Introduction
Dental caries is an irreversible microbial disease of the calcified tissues of the teeth, characterized by demineralization of the inorganic portion and destruction of the organic substance of the tooth, which often leads to cavitation.1 For caries to occur it requires four components namely a) Susceptible host, b) Cariogenic flora, c) Suitable substrate and d) Sufficient length of time. Attempts had been made to prevent caries by a) Increasing the resistance of the host, b) Lowering the number of microorganisms in contact with the tooth, c) Modifying the substrate by selecting non cariogenic foodstuffs and d) Reducing the time that the substrate is in the mouth. By understanding the immunology of dental caries, vaccines are developed and targeted against the antigenic components of the main causative agent S. mutans. So, by which the occurrence of dental caries will be prevented. 2
Immunology of Oral Cavity
The mouth is colonized by microorganisms from birth and most of them are commensals which can become pathogenic when the host responses are altered. The factors responsible for maintaining oral health are a) Integrity of the mucosa, b) Saliva, c) Gingival crevicular fluid and d) Humoral and cellular immune component.
Humoral immune response:
The cell surface of S. mutans possesses many antigens. The most important of them are proteins with enzyme glucosyl transferase and wall-associated proteins. The cell wall enzyme glucosyl transferase (GTF) is an extracellular enzyme which has been used as an immunogen in rodents resulting in inhibition of S. mutans accumulation and caries reduction. GTF has polysaccharide containing glucose, rhamnose, and sometimes galactose and galactosamine. The cell wall also contains lipoteichoic acid (LTA), which is found in all Gram-positive organisms.These antigens may be responsible for some immunological cross reactions between bacterial species. Purification of antigens from S. mutans has revealed two highly immunogenic proteins. One is designated as antigen A (29000 Da) and the other antigen B (185000 Da). Antigen B has two antigenic determinants and is called ‘antigen’ I/II. Antigen B (and possible I/II) is suggested to cross react with human heart tissue. Antigen I/II is present in all strains of S. mutans and S. sobrinus. Sources of antibodies in saliva are a)IgG antibodies from serum, b) IgA antibodies in saliva or c) Combined effect of serum and salivary components. A remarkable feature of the tooth surface is that it is influenced by both local salivary and systemic immune mechanisms. The division between the two immune mechanisms occurs near the gingival margin which is the only site of the body, where an interphase can be found between the secretory and systemic immune mechanisms. Bacteria on the surface of the teeth may be affected by antibodies of two types, the secretory (salivary) antibodies (sIgA) and the serum antibodies (IgG, Ig M, and IgA), which enter the mouth via the gingival crevice.2
Secretory IgA
It is present in secretions such as tears, milk, sweat and saliva. sIgA antibodies are formed by salivary glands in response to antigens that penetrate the glands via the ducts. Secretory form is dimeric, comprising two molecules of IgA united by a polypeptide ‘secretary component’ together with a shorter functional peptide known as the ‘J-chain.’ sIgA antigen complex does not activate the complement mechanism, and S-IgA does not ‘opsonize’ bacteria to promote phagocytosis by polymorphonuclear leukocytes (PMNLs). Salivary IgA plays an important role in defense of host against colonization of streptococci by agglutination of the organisms.3 sIgA occurs in two sub classes a) IgA1 and b) IgA2. IgA1 is produced against certain oral bacteria like S. sanguis. IgA1 can be cleared by bacterial protease whereas; IgA2 is not cleared by bacterial protease because it lacks a 13-peptide sequence where enzymatic cleavage occurs.
Serum IgG
The antigen is bound by macrophages, then presented to the lymphocytes which triggers the secretion of antibodies by plasma cells. The principal functions of IgG are a) Activation of complement, b) Opsonization and c) Inhibition of antigens with enzymes. In complement activation, antigen–antibody complexes stimulate the complement cascade which releases a chemotactic factor that attracts phagocytic PMNLs, release histamine and cause lysis of susceptible bacteria. Opsonization - coating of foreign particles such as bacteria with IgG results in phagocytosis by PMNLs. Inhibition of antigens with enzymes - inhibition of GTF from S. mutans may occur with serum from immunized animals.
Cellular immune response
Cellular immune response does not play a direct role in the immunology of caries. First, because cells have difficulty functioning in the mouth and second, immunity against bacteria is usually not handled by cellular immune mechanisms unless they are chronic and persistent. Most bacterial infections are handled by secretory immunity (secretory IgA) or by antibody (IgG)-complement - neutrophil axis4. The neutrophil is not always necessary for the latter system to be effective. They modify humoral immune response through helper and suppressor actions of T-cells. They cause inflammation of the gingival tissues increasing the gingival fluid flow and hence facilitate access of IgA and PMNLs to the mouth.
Mechanism of action
Plaque in the cervical region of tooth and on root surfaces is influenced by sIgA, serum immunoglobulins, complement factors and PMNLs from the gingival crevice. IgA, IgG, IgM and C3 can be detected in plaque extracts. Plaque in the fissures and coronal parts of teeth is influenced only by salivary antibodies. Bacteria passing through mouth come in contact with Peyer’s patches located along intestinal walls. The T cells and B cells in Peyer’s patches become sensitized to these microorganisms. These sensitized T and B cells migrate through lymphatics to blood stream and settle in salivary glands. They produce IgA that are secreted in the saliva, which are capable of agglutination of oral bacteria and thereby reduce adherence. 2, 3, 4, 5
Antigenic Targets
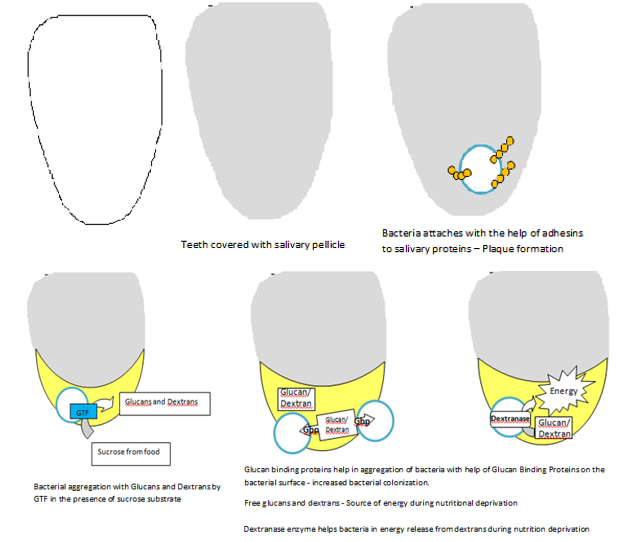
Dental caries vaccines are developed in such a way that they target the antigenic components of S. mutans like a) Adhesins, b) Glucosyl transferase, c) Glucan binding protein and d) Dextranases. (Figure 1)
Adhesins
These are polypeptides that have been obtained from S. mutans and S. sobrinus in the form of intact proteins. Studies reveal that antibody specific for S. mutans Ag I/II or S. sobrinus can interfere with bacterial adherence and dental caries. Active immunization with intact antigen I/II or passive immunization with antibody can protect humans from dental caries caused by S. mutans. 3
Glucosyl transferase (GTF)
The predominant acid produced by most acidogenic bacteria is lactic acid that facilitate demineralization of enamel. Lactobacillus and Streptococcus are the two lactic acid producing bacteria associated with caries. Lactobacillus is associated with dentinal caries while streptococcus is predominant in enamel caries. Another property of these lactic acid bacteria is they utilize extracellular sucrose to form extracellular glucose polymers (glucans) via glucosyltransferase enzyme. S. mutans that have lost the ability to produce GTF are unable to produce disease in animals. Antibody directed against GTF have shown to interfere with the synthetic activity of the enzyme and with in-vitro plaque formation.6 The two major cariogenic streptococcal species in humans are S. mutans and S. sobrinus. Hence immunization with GTF protein or subunit vaccines from one species can induce protection for the other species.
Glucan binding protein (GBP)
S. mutans secretes proteins associated with the bacterial cell called Glucan Binding Proteins. They bind with extracellular glucans in the dental biofilm thereby promoting cellular aggregation of S. mutans. These glucans are the products of enzymatic synthesis by the S. mutans themselves. S. mutans secretes three distinct proteins with glucan-binding activity: GbpA, GbpB and GbpC. Of the three, antibody to only GbpB has shown to induce a protective immune response to dental caries. 7 Thus, it can be achieved through a subcutaneous injection of GbpB in the salivary gland region or by mucosal application by the intra-nasal route.
Dextranases
Dextran is an important constituent of the early dental plaque so that the bacterium can easily invade dextran rich early plaque. Dextranase is an important enzyme produced by S. mutans that destroys dextran. Dextranase when used as an antigen can prevent colonization of the organism in early dental plaque. 3, 6, 7, 8
D ental Caries Vaccination
Historical perspective
In 1924, Clarke isolated an organism, Streptococcus mutans that he suggested to be from the earliest carious lesions in humans. 9 During 1945-46 McClure and Hewitt showed that bacteria were the potential etiologic agents of dental caries. They also proved that ingestion of a cariogenic diet alone was not enough to produce dental caries. In mid-1960s, correlation between cariogenic microbes and host defence system was reached. In 1965, Thomas B. Tomasi et al demonstrated that the IgA system was the primary specific immunological element in saliva. Thus, these two findings set the stage for dental vaccination approaches targeting a specific pathogen (S. mutans) and a specific humoral immune system (sIgA).
Mechanism of action
In vitro studies have shown that IgG antibodies have inhibitory effect on adherence, glucosyl-transferase and acid production of S. mutans. Parenteral immunization establishes noncariogenic microflora on teeth, which could prevent or delay the colonization of pathogenic S. mutans. Few authors suggest that IgA could interfere with establishment of S. mutans and protect against caries because IgA deficient subjects show higher levels of caries than normal controls. Also subjects who showed elevated IgM levels in saliva had a similar caries experience as healthy controls. This indicates a possible protective potential of IgM.
Vaccine designing
Originally, the most popular type of vaccine was prepared from whole cells of killed S. mutans which were used as antigens. Since S. mutans possess antigens which are cross-reactive with heart muscle (heart cross reactive antigens – HCRA), whole cells of S. mutans were not acceptable as parenteral antigens and were orally administered. Therefore, it is of crucial importance to use an alternative means of vaccination which includes a) Purification of the candidate antigens and use of a subunit vaccine, b) Using recombinant DNA methods to place virulence factors from cariogenic organisms into a noncariogenic, non-cross-reactive bacterium.
Routes of vaccine administration
Four routes of immunization have been used with S. mutans are a) Oral, b) Systemic (subcutaneous), c) Active gingivo‑salivary and d)Passive dental immunization.
Common mucosal immune system
As sIgA constitutes a major immune component of major and minor salivary gland secretions, mucosal applications of dental caries vaccine are generally preferred for the induction of secretory IgA antibody. Many investigations have shown that exposure of an antigen to a mucosal-associated lymphoid tissue in the gut, nasal, bronchial, or rectal site can give rise to immune responses. Thus, the concept of “common mucosal immune system” was given by Mestecky.
Oral route
In studies on oral induction of immunity in the gut-associated lymphoid tissues (GALT), antigen was applied by oral feeding, gastric intubation, or in vaccine-containing capsules or liposomes. Animal trials by administering germ-free rats with killed S. mutans in drinking water resulted in significant reduction of caries and increased levels of salivary IgA antibodies. Oral immunization with capsule containing 500mg of GTF from S. mutans also resulted in elevating salivary IgA antibodies.
Intranasal route
Intranasal installation of the antigen, that targets the nasal‑associated lymphoid tissue (NALT), has been used to induce immunity to streptococcal antigens. Protective immunity after infection with cariogenic mutans streptococci could be induced in rats by the intranasal route with S. mutans antigens. Protection could be demonstrated with Ag I/II and GbpB from S. mutans either alone or combined with mucosal adjuvants.
Tonsillar route
Topical application of killed S. sobrinus cells in rabbits induced immune response, which significantly decreased the infection with cariogenic S. sobrinus. 4, 5, 6, 7, 8, 9, 10 Repeated tonsillar application of the antigen induced the appearance of IgA antibodies producing cells in both the major and minor salivary glands of the rabbit.
Minor salivary gland
The minor salivary glands target the lips, cheeks and soft palate as these glands are the potential routes for mucosal induction of salivary immune responses. Experiments with labial application of S.sobrinus GTF had a significantly lower proportion of S. mutans flora in a period of 6‑weeks following a dental prophylaxis, compared with the placebo group.
Rectal
Rectal immunization with non oral bacterial antigens such as Helicobacter pylori, resulted in the appearance of sIgA antibodies in distant salivary sites.11 Preliminary studies have indicated that this route could also be used to induce salivary IgA responses to GTF.
Systemic route
Subcutaneous administration of whole killed S. mutans was used successfully in monkeys and elicited serum IgG, IgM, and IgA antibodies. The antibodies find their way into the oral cavity through gingival crevicular fluid and are protective against dental caries.12 Protection against caries was associated predominantly with increased serum IgG antibodies.
Active gingivo-salivary route
The gingival crevicular fluid has been used as the route of administration which was associated with increased IgG and IgA levels. The various experiments tried were a) Injecting lysozyme into rabbit gingival, which elicited local antibodies from cell response b) Brushing live S. mutans onto the gingiva of rhesus monkeys, which failed to induce antibody formation. c) Using smaller molecular weight Streptococci antigen which resulted in better immune performance probably due to better penetration.
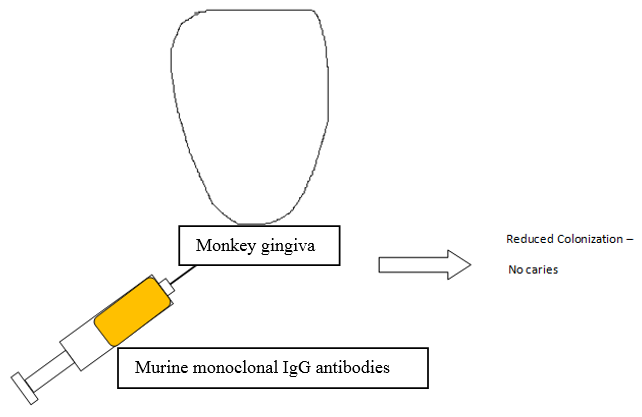
Passive immunization
Passive immunization can be achieved by a)Topical application of murine monoclonal IgG antibodies 13 specific for S. mutans’ antigens to monkey gingiva resulted in decreased colonization by S. mutans and no dental caries over a period of 1 year in contrast to control animals.(Figure 2)
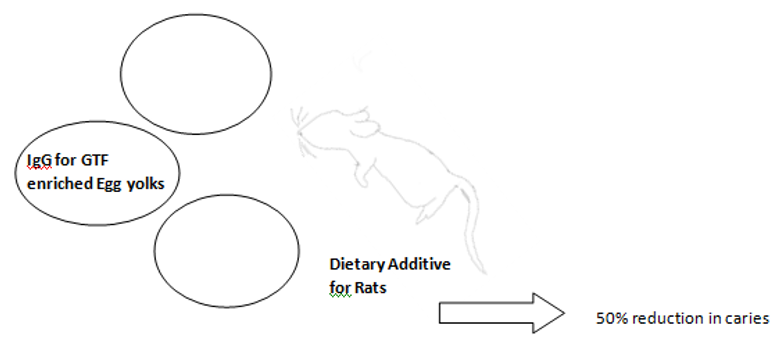
b): Egg yolk antibodies have also been used as a means of passive immunization. This method involved immunization of hens with the GTF antigens and obtaining egg yolks enriched with IgG antibodies for GTF. Experimental use of this antibody enriched egg yolk as a dietary additive in rodent decreased dental caries by 50% (Figure 3)
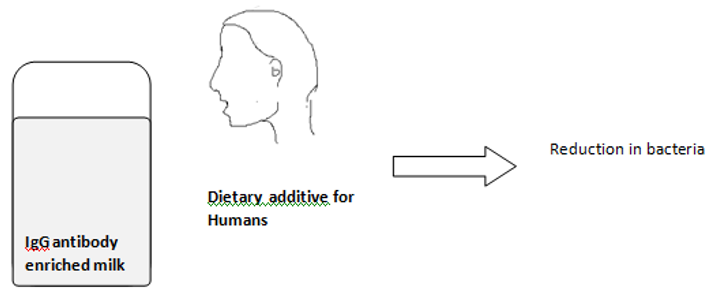
c) Another method includes the use of Immune bovine milk and whey. Systemic immunization of cows with a vaccine from whole mutans streptococcal cells generated IgG antibodies in both the serum and milk whey. In a preliminary human experiment, 14 days use of a bovine milk whey mouth rinse containing antibodies to mutans14 streptococci resulted in a lower percentage of S. mutans as compared with the control group. (Figure 4)
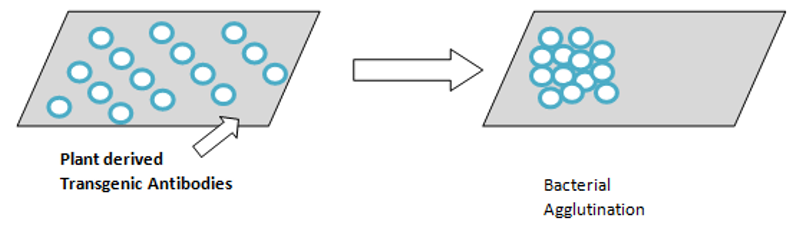
d) Transgenic plant antibodies: Secretary IgA has recently been produced by cross breeding four tobacco plants and the vaccine obtained is colorless and tasteless which can be painted onto the teeth. This antibody showed to be effective against S. mutans and is capable of agglutination of the cells and is the first plant derived vaccine.(Figure 5)
Active immunization
Active immunization can be achieved by a) Ingestion of whole S. mutans in capsule form which do not release its contents until the capsules reached Peyer’s patches, 15 after which it stimulated an antibody response, (Figure 6)
b) Synthetic peptides derived from GTF enzyme from S. mutans as an oral vaccine in rats has shown to effectively inhibit the enzyme function, c) S. mutans antigens coupled to cholera toxin subunits was effective in inducing excellent immune response and in suppressing S. mutans colonization and reducing caries increasing rate and d) Another method was the use of S. mutans genes fused to virulent salmonella. Attenuated salmonella is getting proven to be an effective vaccine technique.
Conclusion
Despite the decrease in dental caries with the use of fluoride mouth rinses, varnishes and professional cleaning, risk of developing caries is high particularly in children from lower socioeconomic background. Caries vaccine has a role in future as it interferes with the metabolism of the major etiological agent. Thus, integrating the caries vaccine after its development into public health programs could be beneficial in bringing dental caries to a minimal level.