Introduction
Periodontal diseases are chronic inflammatory diseases of the supporting tissues of the teeth caused by specific microorganisms or groups of specific microorganisms, resulting in progressive destruction of the periodontal ligament, alveolar bone with pocket formation, recession or both.1 Periodontitis arises due to the aberrant host reaction to a pathogenic biofilm. Neutrophils are the essential gamers of inflammatory and immunological background and are involved in periodontal inflammatory response.2 Neutrophils, the most considerable leukocytes accounting for 50-70% of all circulating white blood cells, and are flexible and unique cells. These are myeloid-derived, expert anti-microbial phagocytes that also can cause death of pathogens extracellularly, linked the innate and adaptive modes of the immune reaction, and facilitates to promote inflammatory resolution and tissue healing.3 The first proof of an organism’s ability to fend off disease came from the research of Russian zoologist, Elie Metchnikoff in the year 1882.4 They are the primary line of defense, i.e. the primary cells to be recruited to sites of inflammation. These cells have a couple of roles to hold the frame of body homeostasis which includes phagocytosis, manufacturing of reactive oxygen metabolites, and degranulation of cytotoxic proteins.5
Neutrophils are the crucial part of immunity response system, abundantly found in blood circulation. They are number one responder to the damage or intruding pathogens in human body.6 PMNs takes part in numerous essential features of the innate immune system consisting of phagocytosis and killing of microorganisms, extracellular matrix degradation, and post-inflammatory healing of tissue homeostasis. It is usually accepted that PMNs recruitment and microbicidal features are important for the preservation of periodontal health.7 Nowadays it’s miles identified that neutrophils are transcriptionally actively complicated cells that produces cytokines, modulate the activities of neighbouring cells and make contribution to the resolution of inflammation.8 These cells have an out of date idea as a “terminally differentiated short- lived, cell without any of transcriptional activities” which underestimated the couple of and wonderful functional capacities of this cell type.9
Approximately, 1 million of neutrophils enter the oral cavity through gingival crevicular fluid in each minute. Neutrophils within the gingival crevice offers the primary cellular host mechanism to manipulate periodontal microorganisms. They packed inside in all of their cellular compartments (like Calprotectin complex, Lysozyme, defensins, cofactor, bactericidal/permeability increasing protein, myeloperoxidase and NADPH oxidase enzyme).10 PMNs appearance in the epithelium is the result of the presence or generation of chemotactic factor in the gingival sulcus and underlying tissues Interaction of PMNs with microorganisms has been proven to harm a variety of cell types, consisting of, fibroblasts, endothelial cells and keratinocytes.11
Neutrophils predominate withinside the early periodontal lesions that characterize gingivitis; however, the relative percentage of neutrophils withinside the inflammatory infiltrate decreases for the duration of transition to periodontitis (Kinane et al. 2008), wherein plasma cells and lymphocytes are dominant. It became to be pronounced that plasma cells occupied 31% of the periodontitis lesion volume, even as the percentage of lymphocytes varied among 5% and 10%. Macrophages and neutrophils have been located in densities of 1–2% and fibroblasts in 5% (Berglundh & Donati 2005). Neutrophils can be especially essential for the duration of transition from gingivitis to periodontitis. Furthermore, they will play a key position in the pathogenesis of periodontitis in the gingival crevice and in the epithelium, wherein they may be the dominant inflammatory cell. The early, non-specific neutrophil response to dental plaque organisms (each pathogenic and non-pathogenic) allows pathogenic microorganisms, together with Porphyromonas gingivalis, to proliferate and invade, putting the level for the transition from gingivitis to periodontitis.12 This relationship of neutrophils to periodontal tissues is equivalent to the proverbial double-edged sword.
Three line of evidence supporting central role of neutrophils in host reponse:13
1st line of evidence
This is derived from clinical studies of naturally occurring diseases involving primary and secondary immunodeficiencies. Neutrophils are the predominant phagocytes in blood. Various studies have shown that defective neutrophil function is associated with presence of periodontal destruction. This was reported in as early in 1902 by Brown PK. Periodontal destruction has been associated with range of neutrophil disorders both induced and innate, including drug, radiation, disease, auto immune-induced neutropenia’s.
2nd line of evidence
This implicates neutrophils as a major protective cell against oral pathogens by observation that several periodontopathic bacteria have significant anti- neutrophil virulence factor. P. gingivalis and A. actinomycetemcomitans are leuko-aggressive i.e. they produce toxins and other factors, which either reduce neutrophil function or kill neutrophils. Moreover, virulence factors which are important in certain periodontal disease, functions by obstructing normal neutrophil function. 14
3rd line of evidence
This comes from the identification of PMN dysfunction in several forms of early onset periodontitis including LJP, rapidly progressive periodontitis and pre- pubertal periodontitis. Neutrophil dysfunctions in LJP include anomalies in chemotaxis, superoxide generation, phagocytosis, and bacterial activity.
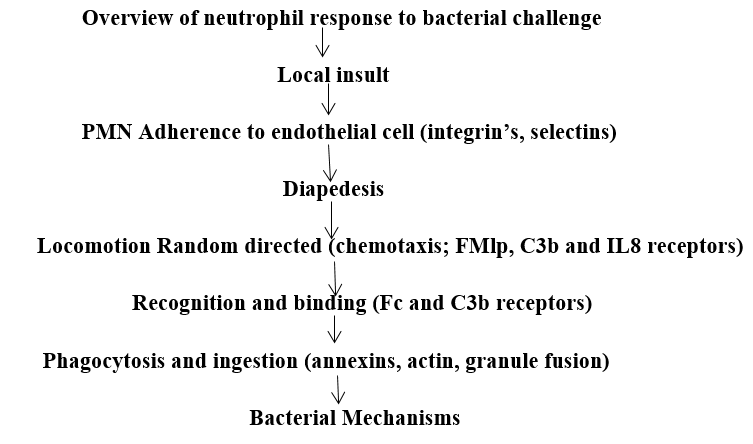
These three lines of evidence strongly suggest that normal neutrophil function is an important determinant of host resistance to periodontal destruction. Neutrophil respond to bacterial challenge in a defined way of molecular events. Any defect in these steps may cause neutrophil dysfunction and make the host more susceptible to bacterial infection. Periodontal neutrophils may play a key role in the modulation of the bacterial ecology of the root surface of teeth and preventing bacterial progression into the junctional epithelium. Short-term experimental neutropenia leads to a rapid apical extension of the subgingival plaque front into the junctional epithelium without the frank bacterial invasion of the subjacent connective tissues.
Specific(secondary) granule mobilization fusion (lysozyme, lactoferrin).
Azurophilic(primary) granule fusion (enzymes, cationic proteins).
Any defect in these steps may cause neutrophil dysfunction and make the host more susceptible to bacterial infection. Disease state involving quantitative and qualitative anomalies of PMN function in many of these mechanisms have been identified. Although the intrinsic functional defect present in each disease state is different, severe periodontitis is a consistent finding of all the conditions.
Why are neutrophils important in periodontal infection? 15
Initially when periodontal pathogens invade, they encounter plasma factors like complement and there is initiation pf inflammation. If complement is not able to control the pathogen, neutrophils come. If neutrophils are also not able to control the pathogen, monocytes are recruited (they develop into macrophages) which either digest the antigen completely or present antigen to lymphocytes. Hence to avoid systemic infection, chronic periodontal inflammation may produce localized specific immune response and a cytokine- controlled amputation of connective tissue called as periodontal disease’. Thus, neutrophils are important because the control the periodontal micro ecology prior to the involvement of chronic inflammatory cells. The concentration of neutrophils in periodontal tissues exceeds the concentration neutrophil in blood. They form a “leukocyte wall” interposed between the plaque mass and the junctional and sulcular epithelium. This wall functions both as secretory and as digestive organ.
Nonoxidative systems may play an important role in influencing the oral microecology by mechanisms acting upon bacterial attachment. One lysosomal component which affects bacterial adherence to in vitro tooth models was lysozyme. It was believed that the effects of lysozyme on adherence could be attributed to its polycationic nature rather than enzymatic activity, although this was not demonstrated conclusively. Recently, Cimasoni et al. reported that neutrophil elastase or cathepsin G decreased the adherence of Streptococcus sanguis but increased the adherence of Porphyromonas gingivalis to saliva-coated hydroxyapatite. Although both neutral serine proteases bound to hydroxyapatite in active form, it was unclear as to whether enzymatic activity was responsible for the observed effects. Similarly, these lysosomal constituents affected the attachment of oral treponemes. The neutrophil may influence bacterial retention. Wilton found that neutrophils or neutrophil lysosomal enzymes were capable of detaching S. mutans from glass. We have observed that intact neutrophils could detach Actinomyces viscosus from saliva-coated hydroxyapatite by an azide-insensitive, energy-dependent mechanism.
Oxidative antimicrobial systems may be important in modulating bacterial colonization of the tooth surface. It may be significant that Charon et al.16 observed higher plaque and gingivitis scores in patients with CGD. We speculated that oxygen metabolism may be important in regulating bacterial colonization. The MPO-H2O2-Cl- system blocked adherence of both A. viscosus and oral streptococci to saliva-coated hydroxyapatite. H2O2 can be derived from either host leukocytes or catalase-negative facultative bacteria such as streptococci. Enzyme activity was absolutely required for these effects to be observed.
In addition to their role in phagocytosis and microbial killing, PMN are recognized to be capable of de novo biosynthesis of several cytokines, including IL-1α and β, TNF-α, and IL-6. Hedley TM et al demonstrated that oral polymorphonuclear leukocytes (PMN) obtained from healthy subject’s express mRNA for IL-1β and release the cytokine in unstimulated culture. Neutrophils are the first line of defense against microbial challenge and their virulence factors. Host response, once initiated by the bacterial stimuli, exerts important effects on connective tissue and bone metabolism. Clinical signs of disease are the response of the body to these sequelae of events. As with the other components of the host inflammatory response, neutrophil functions are also under the effect of several risk factors.
Role of Nneutrophils in Gingivitis
Local response
It is the response to the microflora which is present in plaque. It consists of vascular response with accelerated fluid accumulation and inflammatory cell infiltrate. The early response is especially T-lymphocytic. This response in neighbourhood tissues is not always related with microflora in tissues, however, products that seems to transverse the gingival epithelium, which has misplaced a number of its innate protective barrier functions. The early lymphocytic infiltrate is ruled with the aid of using T- cells however subsequently B-cells become dominant the established lesion is characterized with the aid of using B-cells that have converted into plasma cells in the connective tissue.17
Acute phase proteins which include α2- macroglobulin, α 1-anti trypsin and transferring are accelerated with inflammation reflecting the locally stressed environment. Cleavage of C3, via the alternative pathway is accelerated in GCF in gingivitis. Increased level of activity of IL-1 are observed in chronic gingivitis.18
Kinane et al. showed that levels of IL-1 are increased in GCF which increase in plaque and peak levels precede the clinical signs of inflammation in experimental gingivitis.19
Higher levels of immune mediators like PGE2 LTC4) 1L-B are observed in gingivitis than in healthy sites. Grbic et al. verified that IgA levels are substantially accelerated in GCF from gingivitis while as compared to periodontitis sites, suggesting the ability for IgA to function as a protective factor in this local environment.20
Systemic response
Levels of T-cells responses are barely better in gingivitis it has been recommended that antigens from accelerated bacterial accumulation have greater access to the systemic movement through a break in the integrity of epithelium. Serum antibodies are also accelerated in response to bacterial accumulation.
Role of neutrophils in chronic periodontitis
Periodontitis involves clinically detectable levels of host tissue destruction with clinical attachment loss, periodontal pockets and alveolar bone loss. Increased levels of various microorganisms like P. gingivalis, B. forsthus and P. intermedia, C. rectus, T. denticola, F. nucleatum, A. actinomycetemcomitans are found. Synergistic interactions are found between microbial pathogens. for e.g., enhanced virulence with simultaneous infection with P. gingivalis and F. nucleatum. Formation of pus(abscess) with simultaneous infection with B. forsthus and P. gingivalis / F. nucleatum. 17
Several in vitro studies have demonstrated the presence of hyper-reactive neutrophils in patients with chronic periodontitis, which may lead to an excessive local release of reactive oxygen species and proteolytic enzymes, resulting in periodontal tissue destruction. 21 Several studies have shown increased number of neutrophils- derived substances such as β- glucuronidase (Lamster et al.1994), neutrophils collagenase (Overall et al.1987) and elastase (Gustafsson et al.1992, Meyer et al.1997) in gingival crevicular fluid. Higher amount of elastase could be due to an increase in the number cells and/or to an increased release from each neutrophil. 22
Local response
Various mononuclear cells are present in gingival tissue including plasma cells, lymphocytes, macrophages. The plasma cells are pre-dominated by IgA followed by IgA and no IgM cells in the tissues. IgG cells are identified as IgG>IgG2>IgG3>IgG4> and IgA1 with high IgA2 in the advanced lesions. 23
The TH/ Ts ratio is reduced in chronic periodontal lesion when compared to peripheral blood and chronic gingivitis. Lower TH/ Ts ratio, low response to mitogen and higher expression of HLA-DR on CD8+ T cells have been suggested to be regulators of disease progression in periodontitis. 24 PGE2 levels are increased in periodontitis when compared to healthy sites and, active sites show even higher levels.
With respect to IL-1, studies25 have shown that
IL-1α and IL-1β activity ids >70% in GCF.
IL-1β levels are higher in GCF of periodontitis patients and in active sites when compared to inactive sites.
IL-1β levels and cells producing this cytokine are higher in tissues with periodontitis and are detected in the lamina propria.
IL-6 production has been studied as regulator for B-cell expansion and it has been shown that IL-6 producing mononuclear cells are increased in inflamed tissues of chronic periodontitis and they produce increased amount of IL-6.26, 27, 28 Levels of IL-6 are correlated with bleeding and pocket depth and levels are higher in active disease sites. TNF-α and TNF-β are present in GCF and tissues of periodontitis. IgG levels are increased in GCF from active sites and IgG1and IgG4 are specifically elevated in GCF from active sites of chronic periodontitis. Chronic periodontitis is characterized primarily as involving alternative pathway activation complement with C3 and B cleavage in GCF. Collagenase activity associated with chronic periodontitis is six-fold greater than that of gingivitis. Most of the collagenase activity is due to neutrophil collagenase MMP-8.17
Systemic response
The autologous mixed leukocyte reaction (AMLR) as an indicator of T-cell function to be altered both high and low in chronic periodontitis patient, with circulating CD4+ T0cells significantly reduced in those patients with depressed AMLR and IL-2 generation. The AMLR and T-cell phenotype are restored to normal following successful treatment. Systemic antibody levels are increased in chronic periodontitis patient. There is increased level of serum IgG antibody to P. gingivalis and both IgM and IgA are increased in chronic periodontitis patient.
Role of neutrophils in Aggressive periodontitis
Primary feature of Aggressive periodontitis is the rapid progression of attachment and bone loss. Other capabilities are that patients are otherwise healthy& display a familial pattern of occurrence. There is presence of extended levels of A. actinomycetemcomitans phagocytic abnormalities, hyper responsive monocytes/ macrophages, elevated level of PGE2 and IL-1β. Bacterial killing may be done through numerous serum –mediated mechanism like membrane attack complex of complement, anti-bacterial substances. Several microorganisms like few strains of A. actinomycetemcomitans are resistant to serum mediated killing mechanisms. For serum –resistant bacteria, the neutrophil is the number one host response mechanism of bacterial control.
Rapidly Progressive Periodontitis (RPP)
While neutrophil disorder in LJP has been widely known, only some reviews and case reports have appeared concerning rapidly progressive periodontitis (RPP) (Altman et aL, 1982, 1985; Page et al, 1985). 29 Altman et al (1985) have validated defective chemotaxis of neutrophils and monocytes in few patients with RPP compared with those of periodontally healthy subjects. Page et al (1985) have additionally proven suppressed chemotaxis of each cell at a certain concentration variety of N-formyl methionyl- leucyl-phenylalanine (FMLP). In addition, enhanced random migration has been seen in neutrophils and monocytes of RPP patients. Thus, abnormalities of leukocyte motility in some patients with RPP were proven. The status of other neutrophil functions consists of phagocytosis, intracellular killing, and lysosomal enzyme secretion in RPP remains unclear, even though the functions additionally play a critical role in host defense.
Neutrophil’s phagocytosis in RPP patients is markedly depressed. In the process of phagocytosis through neutrophils, there are two major phases, bacterial attachment to the neutrophil surface and bacterial internalization. RPP patients revealed defective functions in both phases. It has been proven that IgG Fc and C3b receptors of neutrophils participate in every phase (Scribner and Fahrney, 1976). It was suggested that depressed neutrophils phagocytosis in RPP patients was associated with minimum Fc receptors. On the alternative aspect, it has been said that adult periodontitis (AP) patients do now no longer show off those abnormalities of neutrophil functions (Van Dyke et al, 1980; Altman et al, 1982, 1985; Ellegaard et al, 1984; Genco and Slots, 1984; Van Dyke et al, 1980).
RPP is an early- onset, aggressive form of periodontal disease (EOP) that resembles generalized juvenile periodontitis in numerous features. The preceding research established that peripheral blood PMNs from patients with RPP contained significantly higher intracellular quantities of b-glucuronidase, a featured enzyme of azurophil (primary) lysosomes, than the PMNs of healthy controls. Increased manufacturing of azurophil lysosomes though out the promyelocyte degree of granulopoiesis or fewer next diluting mitoses ought to account for the growth of enzymes. The RPP PMNs additionally released much higher amounts of lysosomal enzymes extracellularly in response to an antigenic challenge. It turned into hypothesized that massive growth in extracellular lysosomal enzymes and immunoreactive materials created an ability for an acceleration in the destruction of the periodontium of people with RPP. Interestingly, the accelerated quantities of b-glucuronidase seen in the RPP groups of the earlier investigations appear to be an intrinsic phenomenon, now no longer prompted through the microbial load of the oral contamination of RPP. Granulopoiesis did not appear to be affected by the clinical disease state.
Previous research confirmed that upon stimulation RPP PMNs released higher concentrations of b-glucuronidase, a feature of azurophil enzyme. The inferred concomitant release of different robust azurophil proteases and reactive oxidative metabolites confirmed that RPP PMNs may also have an improved ability potential for destructive proteolysis of the extracellular matrix.30
Prepubertal periodontitis
Prepubertal periodontitis is a disease which impacts the deciduous teeth of young children. Generalized prepubertal periodontitis is frequently related with severe congenital defects of haematological and/or immunological origin. Papllion-LeFevre syndrome, histiocytosis X, Chediak- Higashi syndrome, hypophosphatasia, neutropenia, and mainly Lazy Leukocyte adhesion defects are widely known to be related with early onset periodontitis. Robert & Walker 1976, Shurin et al. 1979, Bowen et al. 1982 describe leukocyte disorder in children with this disorder. Altman L.C, Page R.C et al, 1985 said seven prepubertal periodontitis patients with some form of chemotaxis abnormality observed in all. The mechanism of abnormal leukocyte function in these patients seems to be absence of a 180,000 Dalton glycoprotein from the leukocytes.31
Localised Aggressive Periodontitis (LAgP)
The best-characterized periodontal disease with impaired neutrophil function is LAgP. Peripheral blood PMNs from LAgP subjects show off an accelerated adhesion. Reduced chemotaxis happens in 70% to 75% of cases. There is a decrease in the levels of the surface glycoprotein GP 110 which is related to an altered chemotaxis in LAgP patients. Phagocytosis and killing additionally look like an abnormal shape (bizarre shape). Perhaps the greater applicable commentary with reference to host tissue damage is that superoxide generation has been proven to be extended both in resting and stimulated cells.
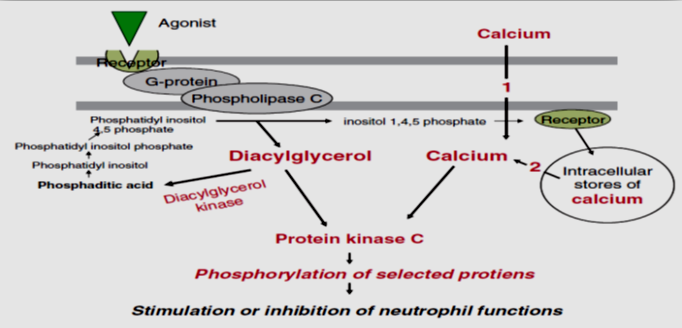
In order to apprehend the mechanisms, that are associated with the onset and outcomes of these abnormal neutrophil functions (bizarre shape) in LAgP, research validated numerous signal transduction anomalies. In neutrophils, chemotaxis and phagocytosis are modulated through a number of receptors and contain numerous activation pathways; the position of intracellular calcium as a presumptive second messenger and mediator of those activities is well established. The effector mechanisms for the chemotactic receptor of neutrophils additionally consists of the activation of phospholipase C and D, protein kinase C (PKC), methyltransferase, or adenylate cyclase. In normal neutrophils, a phosphatidylcholine pathway initiated through phospholipase D, bring about activation of PKC through diacylglycerol (DAG) and the generation of myoinositol-1,4,5 – triphosphate (IP3). In order to deliver the tiers of neutrophil transduction, the usage of fluorescent probes and monitored intracellular calcium adjustment in neutrophils outcomes suggest that the early phase of the calcium reaction affiliated with the discharge of intracellularly sequestered calcium seems intact in LAgP neutrophils. The second phase of the calcium response, related to membrane channel activation and an inflow of extracellular calcium, seems compromised within side the neutrophils of the LAgP population. Furthermore, DANIEL et al. additionally have proven that acceleration in the intracellular calcium (Ca2+) levels that is a normal end result of activation of myoinositol 1, 4, 5-triphosphate pathway, does now no longer arise in LAgP patients.32
There is a strong association between defective chemotaxis and reduced intracellular Ca2+ levels. Recent facts from their group of institution have mentioned the calcium influx factor (CIF) which acts as a second messenger inducing Ca2+ access, is appreciably decreased in LAgP patients. CIF is a second messenger in calcium store depletion – prompted calcium access. This mechanism for calcium access performs a main position in neutrophils. Reduced CIF content in LAgP neutrophils indicate that CIF activity can be critical determinant in disorders of those cells. Taken collectively with the finding of depressed chemotactic response, those fact in addition suggest that abnormal (bizarre) chemotaxis in neutrophils can be associated with the availability of CIF.
Table 1
Functional abnormalities in localized aggressive periodontitis (LAgP)
PKC is a key molecular factor in neutrophil signal transduction after stimulating the receptors via soluble bioactive molecules. To similarly make clear the mechanism of this altered response and to affirm and increase in advance observations, the calcium – established PKC activity of neutrophils from patients with LAgP was evaluated.33
In LAgP patients, the overall calcium – established PKC activity of neutrophils (201.0 +/- 63.6 pmol / min / 10 cells) changed into decreased than that of neutrophils from healthy subjects (287.6 +/- 63.6 pmol / min / 107 cells) and the distinction changed into statistically significant. The PKC activity in neutrophils from patients with LAgP exhibited a positive association with chemotactic migration to N – formyl methionyl-leucyl-phenylalanine (FMLP). The low activity of the PKC in neutrophils from the patients contemplated the low activity within side the soluble fraction from the neutrophils. After stimulation with phorbol 12 – myristate 13 – acetate (PMA), PKC activity was observed to be decrease in patients with LAgP than from healthy subjects. These outcome advices that decrease PKC in neutrophils is a predisposing factor of LAgP.
LAgP neutrophils exhibit showcase a marked increase in diacylglycerol (DAG) in each stimulated and unstimulated cells, and this acceleration is related to pronounced decrease in DAG kinase activity. These observations had been additionally independently confirmed. DAG is a diglyceride and a physiological activator of PKC and functions as a potential second messenger. It has been implicated in the regulation of a variety of cellular functions including neutrophils activation, superoxide production, degranulation, chemotaxis and chemo kinesis. It is metabolized into phosphatidic acid (PA) via DAG kinase (DGK). They also observed that the level of DAG is markedly increased up to 2-Fold in unstimulated peripheral blood neutrophils from patients with localized juvenile periodontitis (LAP) as compared to cells from normal individuals. These cells additionally confirmed an enhanced and prolonged elevation of diglyceride upon stimulation with FMLP. The metabolism of a cell – permanent DAG through DGK was significantly decreased. Analysis of enzyme kinetics discovered a 5 – fold or higher elevation in an apparent Km of cellular diglyceride kinase. Simultaneously also observing an elevation of DAG levels and decreased DGK activity suggests that the LAgP neutrophils exist in a primed or preactivated state.
More recently, they studied the mechanism for the reduction in DGK in LAgP neutrophils to decide whether or not this can be associated with a mutation, post-translation modification, differential expression, or loss of expression of a specific isoform (s). In this work, mRNAs for the diverse isoforms of DGK in normal and LAgP neutrophils had been analysed, and alpha, beta and gamma isoforms had been diagnosed through polymerase chain reaction using unique oligonucleotide premises for every isoform. No major variations had been observed in the isoform pattern among unstimulated normal and LAgP neutrophils. However, the stages of mRNA for the alpha and beta-isoforms of DGK had been improved in normal neutrophils, at the same time there is a barely decreased in LAgP upon stimulation with FMLP. These findings suggest that alterations in the levels of mRNAs for diverse isoforms of DGK in the course of cell stimulation can be a crucial issue in expertise the molecular defects related to LAgP.34
Formylpeptide receptors (FPRs; 55,000 per cell) are found on the surface of PMNs and other phagocytes. Like other G-protein–coupled receptors, the FPR contains seven transmembrane domains separated by extracellular or intracellular loops. Formyl- Met-Leu-Phe(fMLF) and other formylpeptides are produced by bacteria and occur at high concentrations at infection sites. fMLF binding to these receptors triggers a cascade of intracellular signals that coordinate cytoskeletal reorganization, formation of pseudopodia, and migration toward a chemotactic gradient. PMNs from patients with AgP exhibit reduced fMLF binding and <20% to 50% of the chemotactic activity toward fMLF observed in healthy control subjects.
The production and release of Nitric Oxide from human neutrophils are also reported. Moreover, NOS activity, NOS mRNA, and NOS protein have been detected in human neutrophils. NO has been shown to activate soluble guanylate cyclase, leading to accumulation of cGMP. Intracellular accumulation of cGMP has been suggested to regulate neutrophils chemotaxis. NO, NOS, and NÉ-nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor, have been suggested to regulate neutrophil chemotaxis and/or be involved in neutrophil chemotaxis. Human oral neutrophils are fully primed and produce larger amounts of NO than peripheral blood neutrophils. The involvement of Aggregatibacter actinomycetemcomitans (A.a.) in the pathogenesis of LAgP has been documented. LPS from A.a. induces significant NO production. This evidence suggests that NO may be involved in cellular reactions in LAgP.
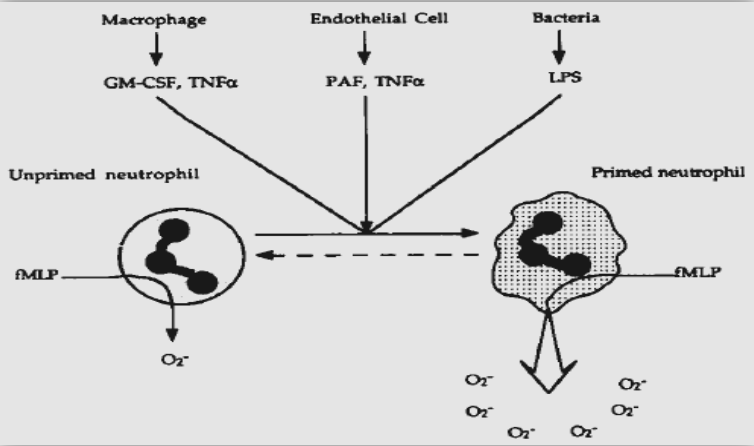
Furthermore, these observations, in conjunction with the increased superoxide production and reduced chemotaxis that are characteristic of primed neutrophils, may be relevant to the fact that PMNs exhibit functional abnormalities due to their priming, unrelated to any major deficiency of signalling molecules.
Van Dyke et al. have summarized the major findings of Polymorphonuclear leucocytes defects in Localized Aggressive Periodontitis as follows:
Random migration and deformability are normal; adherence is normal or slightly elevated
In 75% of the Localized Aggressive Periodontitis patients examined for a variety of chemotactic factors, chemotaxis has been depressed due to a lack of response to the chemotactic gradient. The defect is mostly intrinsic to the Polymorphonuclear leucocytes cell, (Lavine et al 1979) long lasting, Clark et al 1979 reported that this defect was not reversed by periodontal therapy and found also in healthy siblings.
The Localized Aggressive Periodontitis Polymorphonuclear leucocytes have a reduced number of binding sites for the chemotactic peptide formyl-methionyl-leucyl-phenylalanine (fMLP).
Van Dyke et al. have shown that the migratory cytoskeletal apparatus is intact in Localized Aggressive Periodontitis Polymorphonuclear leucocytes. They found no shift to a larger, nonmotile Polymorphonuclear leucocytes subpopulation in Localized Aggressive Periodontitis, although two Polymorphonuclear leucocytes populations with different chemotactic activities have been found in normal human blood.
Generalised Aggressive periodontitis
Patients frequently have a neutrophil chemotactic detect and reduced phagocytosis. The chemotactic defect is cellular in nature, but the neutrophils have normal random migration and oxidative metabolic activity. Although the incidence of chemotactic defects is similar but less in GJP than in LJP, GJP patients are less likely to exhibit decreased phagocytosis. Patient exhibit high IgG antibody titres to Porphyromonas Gingivalis. Antibodies to Aggregatibacter actinomycetemcomitans are less frequent in GJP then in LJP.
Role of neutrophils in refractory periodontitis
Cases that for unknown etiology do not longer respond to therapy and/or recur quickly after adequate treatment have been referred to as refractory periodontitis. Heterogeneity of scientific presentation and disease progression and development of refractory patients was proposed as early as 1985. Two unique refractory disease types have been described: immediate refractory disease onset after treatment and a refractory pattern that develops after a quiescent period. Microbiologic heterogeneity has been recognised amongst refractory patients, and it has been counselled that RP is not a single entity, but it is an association in groups of several periodontal diseases.35 P. gingivalis, B. forsythias, F. nucleatum, P. micros, E. corrodens numbers are increased in patients who do not respond to treatment.
Neutrophils display defective chemotaxis and depressed phagocytic function. Neutrophils from the rapidly aggressive periodontitis patients have an intrinsic hyperreactive pathway from PKC to the NADPH oxidase complex, in evaluation to patients with chronic periodontitis wherein neutrophils are activated through the FcY receptor pathway.
Local Responses
Pathogens related to disease in this group seems to be similar to those in different forms of periodontitis and suggestion has been made that this group represent a host defect in response to bacteria. Sites with refractory periodontitis with the highest total cytokine level demonstrated that IL-6 levels are higher than stable patients. Local IL-1 and IL-6 are produced in response to different factors and IL-6 may play a role in refractory periodontitis
Systemic responses
CD4/CD8 ratio is reduced in refractory periodontitis.
There is an expanded IL-β and PGE2 secretions in comparison to gingivitis or normal subjects.
There is increased in IgG antibody to a couple of pathogens.
There is altered cell-mediated immunity, host defences in patients with an intense form of periodontitis not responding to treatment.
Role of neutrophils in ANUG
Acute necrotizing ulcerative gingivitis-periodontitis (ANUG/ NUP) is a relatively uncommon periodontal disease characterized by gingival necrosis and ulceration, pain, and bleeding. It is a painful and rapidly progressive disease of the free gingiva, attached gingiva, and alveolar mucosa. NUP is a progression of the disease to the supporting tissues of bone and periodontal ligament. Three primary clinical criteria for the diagnosis of ANUG:
Additional clinical criteria that have been seen in ANUG are fetor oris, lymphadenopathy, malaise, fever, and pseudo membrane formation. Ronald B. Cogen et al reported that depression of some host defence mechanisms, particularly PMN chemotaxis and phagocytosis, may be important in the pathogenesis of ANUG. The frequency with which ANUG occurs in disease states which involve depression in numbers and/or functions of leukocytes, such as severe debilitating illness, blood dyscrasias malnutrition, Down’s syndrome, Chediak- Higashi Syndrome and insulin – dependent diabetes has led to the supposition that depression in host defense mechanisms may play a significant role in the etiologic role and severity of ANUG.36
An electron microscopy study of ANUG was first undertaken by Listgarten in 1965 who identified four zones containing spirochetes from tissue sections of ANUG lesions:
Bacterial Zone: The bacterial area with a superficial fibrous mesh composed of degenerated epithelial cells, leukocytes, cellular rests, and a wide variety of bacterial cells, including rods, fusiform, and spirochetes
Neutrophil rich zone: The neutrophil-rich zone composed of a high number of leukocytes, especially neutrophils, and numerous spirochetes of different sizes and other bacterial morphotypes located between the host cells
Necrotic Zone: The necrotic zone, containing disintegrated cells, together with medium- and large-size spirochetes and fusiform bacteria
A zone of spirochaetal infiltration: The spirochaetal infiltration zone, where the tissue components are adequately preserved but are infiltrated with large- and medium-size spirochetes. Other bacterial morphotypes are not found.
Listgarten's electron microscopy study of ANUG lesions found spirochetes predominantly in the necrotic zone and the zone of spirochaetal invasion. The invading spirochetes were primarily large and intermediate in size and were described as morphologically different from Borrelia Vicenti. Low numbers of spirochetes were noted in clinically normal sulci. Intermediate sized spirochetes were predominant in ANUG lesions." Courtoiset ale observed Listgarten's four zones of the ANUG lesion and also noted an inflammatory infiltrate composed of significant numbers of plasma cells and lymphocytes.
Hooper and Seymour stated that the histopathology of ANUG distinguishes it from simple gingivitis. The histopathology picture shows inflammation, ulceration and extensive necrosis of the affected gingival tissue. Light microscopy shows ulceration of the stratified squamous epithelium with a fibrinous pseudo membrane. The pseudo membrane is composed of microorganisms, PMN cells and necrotic tissue debris. Epithelium and connective tissue adjacent to the area of ulceration is heavily infiltrated by polymorphonuclear leukocytes (PMNs). PMN infiltration is associated with widening of the intercellular spaces and destruction of epithelial cells, probably mediated by release of hydrolytic enzymes from PMNs. The connective tissue is filled with dilated blood vessels and, in the deeper layers, an infiltration of plasma. Cells and lymphocytes. The capillary loop network supplying the gingival papillae and interdental col is vulnerable to a reduction in blood flow from a number of causes.
Role of neutrophils in periodontal Abscess
The periodontal abscess has been described as "an acute, inflammatory destructive process in the periodontium which results in localized collections of pus and have communication with the oral cavity through the gingival crevice or any other periodontal sites and may not be arising from the dental pulp. It is also referred to as lateral abscess or parietal abscess.
Pus is a purulent exudate, which may be creamy or opaque in appearance, usually composed of dead and living neutrophils, red blood cells, fragments of tissue debris and fibrin. In mature abscess, macrophages and cholesterol crystals are also present. An abscess is a protective response of the body tissue to prevent the spread of infectious substances to different components of the body. Abscess is coated through pyogenic membrane. The pyogenic membrane is fashioned through the neutrophils and macrophages in a try to maintain the pus from infecting neighbouring structures. However, such membrane has a tendency to save immune cells from attacking microorganisms within side the pus, or from attaining the causative organism or foreign body.
The first step within the improvement of a Periodontal abscess is bacterial invasion of the soft tissues surrounding the periodontal pocket, which will form into an inflammatory manner via the chemotactic elements launched through microorganisms which have the ability to attract polymorphonuclear leukocytes (PMN) and other types of cells. This will trigger intensely release of cytokines; results in destruction of the connective tissues; encapsulation of the bacterial contamination and formation of pus.
Histologically intact neutrophils are located surrounding a central area of soft tissue debris and destroyed leukocytes. At this stage, a pyogenic membrane consists of macrophages and neutrophils which may further organised in the various compartments. An acute inflammatory response surrounds the purulent vicinity, and the overlying epithelium reveals intracellular and extracellular edema and invasion of leukocytes. Once the abscess is developed, the rate of destruction within the abscess will depend on the growth of microorganisms inside the area of foci; their virulence, and the local pH (an acidic surroundings) will favour the activity of lysosomal enzymes.
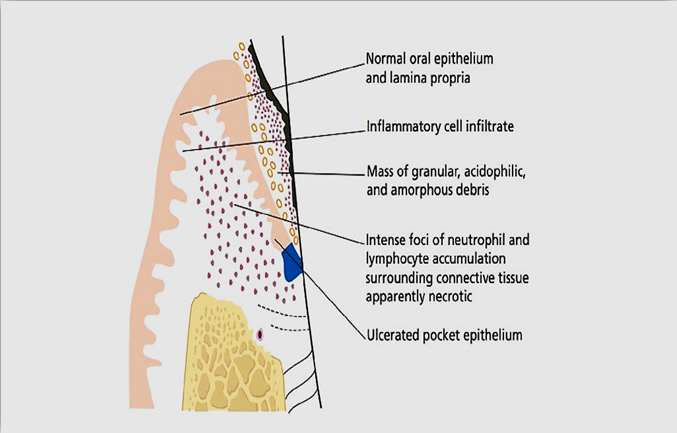
De Witt et al. (1985) investigated the punch biopsies taken from 12 abscesses. The biopsies have been taken just apical to the centre of the abscess. They observed, from the outside to the inside: a) a normal oral epithelium and lamina propria; b) an acute inflammatory infiltrate; c) an intense focus of inflammation (neutrophil lymphocyte) with the encompassing surrounding connective tissue destroyed and necrotic; d) a destroyed and ulcerated pocket epithelium; e) a central or primary region, as a mass of granular, acidophilic, and amorphous debris. In 7 out of 9 specimens evaluated through electron-microscopy, gram-negative anaerobic flora has been visible and further invade the pocket epithelium as well as alteration in the surrounding connective tissue. 37
Laboratory tests will also be used to verify and investigate the diagnosis. The increase in numbers of the blood leukocytes, neutrophils and monocytes can be suggestive of an inflammatory reaction of the body to microbial toxins within side the periodontal abscess.
Periapical Abscess
Periapical abscess is the result of pulpal infection that extends through the apical foramen to the periapical tissue. It may develop fistulous tract that communicates with the oral cavity or may develop a communication with the periodontal pocket or the gingival sulcus. The cytologic characteristics are typical. Neutrophil predominate at the apex and are massively corrugated in the area of the abscess and in ulcerated areas. These cells migrate from dilated blood vessels in an attempt to protect the tissues from invading organisms by their phagocytic and enzymatic actions. The more virulent the bacteria, the greater the leukocytic migration into the affected tissue area. The presence of pus exceeding through a fistula is an indication of this leukocytic activity.
Conclusion
Neutrophils and macrophages are critical in host defense against bacterial infection. When phagocytic cell number or function is compromised, disease progression and severity are markedly increased. Periodontal disease is a common sequela associated with altered phagocyte response.
There is a robust association among altered PMN activity and localized aggressive periodontitis. Altered neutrophil function is an attractive model system for understanding periodontal pathology in LJP.
Cells of the monocyte/macrophage lineage have a pivotal role in control of infection and wound repair. Elucidating the role of monocytes in the resolution of inflammation is crucial for our understanding of periodontal disease.
The great majority of Polymorphonuclear leukocytes (PMNs) are detected both in patients with gingival inflammation and in clinically healthy subjects. It has been suggested that crevicular fluid PMNs at the dental plaque surface contain phagocytosed bacteria and since the function of CF-PMNs is to kill microorganisms, it is probable that these cells protect the gingival tissue from invasion by the organisms of dental plaque. However, ultrastructural studies have demonstrated that CF-PMNs degranulate in diseased gingival tissue and crevicular exudates, resulting in the extracellular discharge of lysosomal granules. This observation raises the possibility that CF-PMNs may serve as a mediator of tissue injury in periodontal diseases.
In summary, while a large body of data implicates neutrophil dysfunction, either intrinsic or acquired, as a significant risk factor for periodontal diseases, clear, prospective, longitudinal epidemiological studies to evaluate this association remain to be performed. As a framework for understanding the role of phagocytic cells in the progression of infectious diseases, human pathology provides evidence for a clear relationship between compromised phagocyte number or function and increased disease incidence and progression. In the case of intrinsic cellular neutrophil anomalies, sufficient evidence has been accumulated to assign a predictive value to the association with periodontal diseases, as well as a causal relationship in the pathogenesis of disease. By contrast, acquired neutrophil abnormalities are essentially in vitro observations and the causal relationship of these secondary neutrophil abnormalities remains speculative.