- Visibility 307 Views
- Downloads 19 Downloads
- DOI 10.18231/j.idjsr.2021.018
-
CrossMark
- Citation
A comparative study of remineralizing efficiency of various remineralizing agents on artificially demineralized enamel surface – An in vitro study
- Author Details:
-
Chandra Sekhar Manduru *
-
Sushma Chandra
-
Gopi Krishna Moosani
-
Nogalakshmi Reddy Sampathi
-
Upendranaha Reddy
-
Anusha Yalamatchi
Introduction
Dental caries remains a major significant public health problem even though the prevalence of the disease has decreased since the introduction of fluorides. The current research in caries has been shifted to the development of methods for the early detection of caries lesions and the non-invasive treatment of these lesions.[1]
Dental caries occurs when the demineralization process exceeds remineralization. The progression of dental caries lesion is a slow process, and during the early stages, non-invasive intervention converts lesion from an active state to an inactive state. The process of caries formation is a cycle of remineralization and demineralization; according to these cycles, it can be either reversible or irreversible process.[2]
When pH drops below 5, there will be the initiation of early enamel caries. The remineralization process opposes demineralization by neutralizing oral pH. Early diagnosis of incipient lesions can lead to a new era in preventive dentistry in the form of remineralization. One of the best modes for caries management is the use of remineralizing products.[3] In the past, there have been many strategies to combat dental caries. During these last ten years, there is an advancement in the reversal of initial carious lesions using a non-invasive treatment like remineralization.[3]
Remineralization is a process where it relies on calcium and phosphate to rebuilt a new surface after demineralization. Reconstruction of the depleted tissues with hydroxyapatite (HA), which is the same inorganic component as the enamel, is one of the ideal methods of increasing remineralization.[4] It has been more than a century for dentistry to advance from an "extension for prevention" concept to a newer concept "minimum intervention." It refers to the principle of treatment, in which early intervention minimizes tooth destruction because the disease is diagnosed before the destruction of the tooth. Hence, it is possible to remineralize the carious lesion. Application of therapeutic agents for remineralization is an inclination towards minimal intervention procedures causing less or no destruction of tooth substance.[5]
Recently, various remineralizing agents are available for treating initial carious lesions. Calcium sucrose phosphate (Enafix) remineralizing agent is available only as a dentifrice. Its quickly breaks down and release calcium, phosphate, and sucrose phosphate ions into the saliva, calcium and phosphate ions, rapidly absorb onto the enamel, decrease the rate of enamel solubility under acidic conditions.[6]
Casein Phosphopeptide–Amorphous Calcium Phosphate (CPP–ACPF) (GC Tooth Mousse, India) was introduced as a remineralizing agent in the year 1998. It contains nanocomplexes of milk protein Casein phosphopeptide with ACP.[7] It has been claimed that it enhances remineralization of the early carious lesions by maintaining a supersaturated environment for essential minerals, at the same time it also hinders colonization of dental surfaces by cariogenic bacteria.[8]
Clinpro tooth creme is a 0.21% w/w sodium fluoride (NaF) anti-caries dentifrice that consists of 950 ppm fluoride and a functionalized tricalcium phosphate (f-TCP). Fluoride combination with TCP not only provides greater remineralization in terms of surface microhardness and fluoride uptake but also decreases the dose of fluoride required to achieve the same degree of RML.[9]
Xylitol (squigle) is a non-toxic sugar alcohol sweetener, and resistant to fermentation by streptococcal caries- inducing bacteria of the plaque. It has become a widely used non-cariogenic food additive. Xylitol is effective in preventing dental caries by inhibiting the growth and metabolism of mutans streptococci. Xylitol forms complexes with calcium ions and it prevents more ample calcium phosphate precipitation, and it can also facilitate the transport of calcium and phosphate ions for remineralization of demineralized enamel.[10]
So, this present study evaluates the remineralizing capacity of various remineralizing agents like calcium sucrose phosphate (Enafix), casein phosphopeptide-amorphous calcium phosphate with fluoride (GC tooth mousse plus), tricalcium phosphate with sodium fluoride (Clinpro tooth creme) and xylitol (Squigle).
Materials and Methods
The study samples were collected from the Department of Oral and Maxillofacial Surgery, G. Pulla Reddy Dental College & Hospital, Kurnool, Andhra Pradesh.
Inclusion criteria
Permanent premolars extracted for the orthodontic purpose were selected. The teeth selected were noncarious, with an intact surface.
Exclusion criteria
Any tooth with visible cracks, hypoplasia, enamel white spot lesion or caries on any surface, and restored teeth was excluded from the study.
Thirty freshly extracted premolar teeth extracted for orthodontic reasons were used. The teeth were decoronated, and the crown portions were sectioned into four segments of two buccal and two palatal halves, each using a double-faced diamond disc mounted on a contra-angle handpiece.
Enamel samples were embedded in self-cure acrylic with the enamel surface exposed. These samples were stored in 10% formalin until further use. A total of 120 enamel samples were divided into six groups of 20 samples each (n=20). ([Figure 1])
Group A - Calcium sucrose phosphate (Enafix).
Group B - CPP-ACPF Containing dentifrices (GC Tooth Mousse plus)
Group C - NaF with tricalcium phosphate (Clinpro Tooth Creme).
Group D - Xylitol (Squigle).
Group E - Positive control.
Group F - Negative control.
The demineralizing solution was made in the Department of Oral Pathology (G.Pullareddy Dental College and Hospital). A digital pH meter was used to check pH during and after the preparation of the demineralizing solution.
The composition of demineralizing solution used in the study was as follows:
2.2 mM calcium chloride, (CaCl₂·2H₂O)
2.2 mM monosodium phosphate, (NaH₂PO₄·7H₂O)
0.05 M lactic acid, (C3H6O3)
The final pH of the solution was adjusted to 4.5, with 50% sodium hydroxide (NaOH).
All the enamel samples belongs to Groups A, B, C, D, and F were immersed into a glass container containing 100 ml of prepared demineralizing solution for a period of 48 h at 37°C inside universal Incubator. This demineralizing process was contemplated to produce a subsurface lesion. After 48 hours of incubation, the teeth were washed with de-ionized water, dried with the help of an air syringe, and placed in their respective glass containers until further evaluation.
The samples in Groups A, B, C, and D were treated with respective remineralizing agents at every 24 h for seven days. Samples were rubbed with respective remineralizing agents with the help of a polishing cup attached to a contra-angle handpiece for 4 min, washed with de-ionized water, and then placed in artificial saliva. ([Figure 2]) All these samples were placed in a universal Incubator at 37°C before each remineralizing cycle. In the control group, samples were only washed with de-ionized water and placed in artificial saliva. Artificial saliva was renewed for every 24 h just before immersion of freshly treated samples.
After seven cycles of remineralization, the surface microhardness of the specimens was determined using Vickers microhardness testing machine (Matsuzawa, kawabe, japan). A load of 100 g was applied to the surface of each specimen for 10 seconds using Vickers elongated diamond pyramid indenter under a 40× objective lens.([Figure 3]) The accuracy of values of the diagonal length of indentations was determined under a high magnification of 400×. The depth of the indentations was measured with built-in scaled microscope, and the values were converted to Vickers microhardness values. ([Figure 4]) Three indentations were placed on the surface, and the average value was considered for each sample.



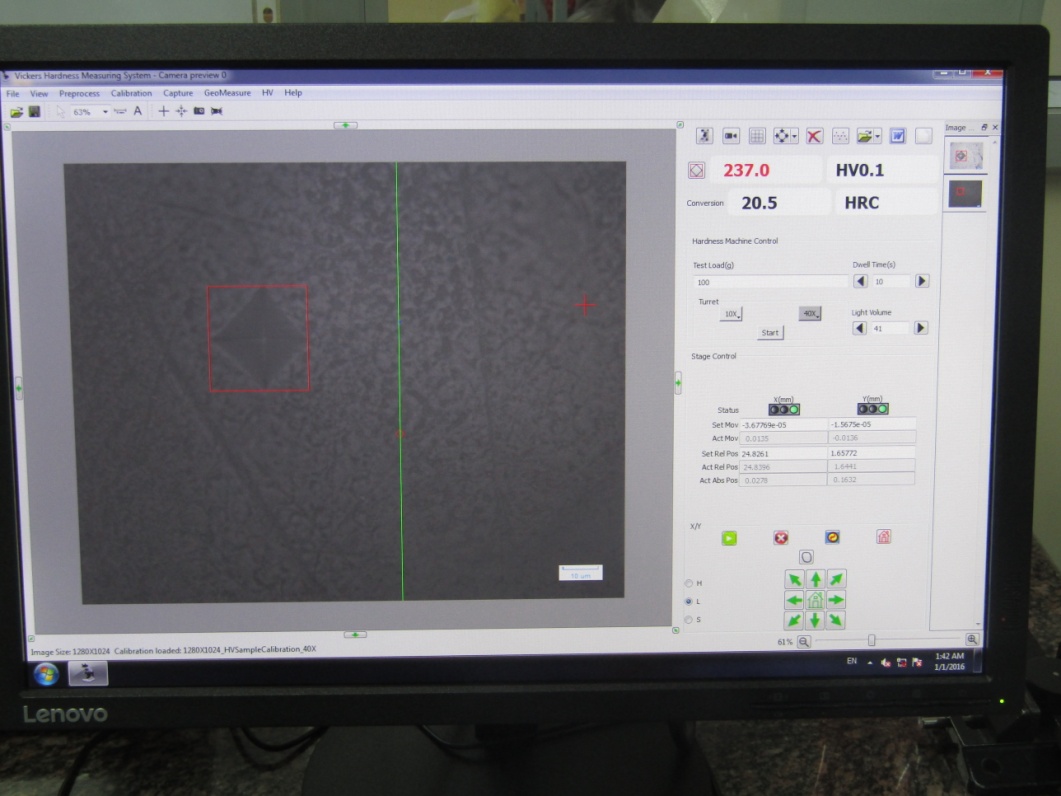
Statistical analysis
The mean and standard deviation of the microhardness of tooth samples were obtained for each group and compared using one-way ANOVA. The pair-wise comparison was carried out using a post hoc Tukey test. The statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA) software.
Observation and Results
The mean for the positive control (Group E) was the highest 260.26 ± 47.69 MPa, while that of the negative control (Group F) was the lowest 187.03 ± 22.76 MPa. Among the materials used Group D (Squigle) showed the highest mean 232.79 ± 41.04 MPa, followed by Group A (Enafix) with a mean value of 226.37± 20.38 MPa, Group B (GC Tooth Mousse) with a mean value of 218.16± 11.90 MPa, Group C (Clinpro Tooth Creme) with least mean value of 214.38± 12.57 MPa. ([Table 1], [Figure 5])
Pair-wise comparison of mean surface microhardness among the groups was done by using post hoc tukey test . Pair-wise comparison of microhardness was performed between groups to determine which groups differed significantly from other. The results showed that the SMH values of Group E (Positive control) was significantly higher than all other groups (P < 0.05). Among the various remineralizing agents used, the mean mcrohardness of Group D (Squigle) was higher than the other three experimental groups; but not statistically significant (P > 0.05). But, its mean differed significantly from that of negative control and positive control (P < 0.05). There was a statistically significant difference when all remineralizing agents compared to the positive and negative control, but there was an insignificant difference between remineralizing agents used. ([Table 2])
|
Parameter |
Frequency |
Mean ± SD |
Mean Square |
F Value |
P value |
|
Group A |
20 |
226.37 ± 20.38 |
11546.322 |
11.242 |
<0.001* |
|
Group B |
20 |
218.16 ± 11.90 |
|||
|
Group C |
20 |
214.38 ± 12.57 |
|||
|
Group D |
20 |
232.79 ± 41.04 |
|||
|
Group E |
20 |
260.26 ± 47.69 |
|||
|
Group F |
20 |
187.03 ± 22.76 |

|
Groups |
Mean Difference |
P-Value |
|
|
Group A |
Group B |
8.20 |
0.950** |
|
|
Group C |
11.99 |
0.790** |
|
|
Group D |
6.42 |
0.983** |
|
|
Group E |
33.88 |
0.005* |
|
|
Group F |
39.33 |
0.001* |
|
Group B |
Group C |
3.78 |
0.999** |
|
|
Group D |
14.63 |
0.618** |
|
|
Group E |
42.09 |
< 0.001* |
|
|
Group F |
31.12 |
0.014* |
|
Group C |
Group D |
18.41 |
0.361** |
|
|
Group E |
45.88 |
< 0.001* |
|
|
Group F |
27.34 |
0.045* |
|
Group D |
Group E |
27.46 |
0.043* |
|
|
Group F |
45.75 |
< 0.001* |
|
Group E |
Group F |
73.22 |
< 0.001* |
Discussion
Despite many advances in the field of dentistry, dental caries remains a significant problem affecting the human population across the globe. However, the caries process is now well-understood; much of it has been described extensively in the dental literature.[11]
Demineralization is the process of loss of mineral from the tooth structure, while remineralization is a gain of mineral in the form of hydroxyapatite to the tooth structure. Remineralizing agents create a supersaturated environment around the early carious lesion; thus, prevents mineral loss and delivers calcium and phosphate ions in the vacant areas. Usually, these agents contain calcium phosphate with or without fluoride. The basic mechanism of remineralization involves the diffusion of calcium and phosphate ions from saliva and other topical sources, which is aided by fluoride ions to build a hypermineralized, fluorapatite, acid-resistant layer on the previous crystal remnants, which act as remineralization nuclei.[12], [13]
In the present study, to simulate oral conditions, the specimens were stored in the demineralizing solution for 48 hours at 37°C in Universal Incubator. It resulted in a subsurface demineralization with an intact surface replicating an early enamel lesion. Artificial created caries-like lesions of enamel are more evenly reproducible than natural lesions, and it acts as a reliable experimental model.[14] Artificial saliva was used as a solvent to dilute the remineralizing agents for the following reasons. It has been reported that saliva enhances the effectiveness of remineralizing agents. This could be related to the ability of artificial saliva to adjust the pH of the remineralizing agent that enhances the remineralization process.[15]
Vickers microhardness test method was used to analyze the demineralized and remineralized dental tissues. It is a relatively simple, rapid, and non-destructive method.[16] Vickers diamond indenters are commonly used. However, it has been reported that the Vickers indenter is more useful than the Knoop because a square shape has to be always conserved; and any small elongation of the diagonals of the indentations that produce errors in hardness measurements can be easily detected. Therefore, the Vickers indenter was used in the current study.[17], [18]
In the present study, the mean microhardness of positive control group was significantly higher than all other groups. Among materials, xylitol with fluoride had the highest remineralization potential (232.79 ± 41.04), when compared to other remineralizing agents. Xylitol is believed to be a non-fermentable, "tooth-friendly," sugar alcohol. The main properties of this sweetener are that it is not fermented to acids, forms less of plaque, and reduces the number of Mutans streptococci in saliva.[19] It acts on the mitochondria of streptococcus mutans and inhibits the process of glycolysis of these microorganisms, thus interfering with their growth and metabolism. Xylitol works by making the pH alkaline, and it stimulates the salivary flow rate, which increases the salivary clearance, buffering power, and degree of saturation with calcium and phosphates thereby, neutralizing the decrease in plaque pH/salivary pH that occurs after meals.[20]
Miake et al. stated that xylitol might accelerate remineralization by lowering the diffusion coefficients of Ca2+ and PO43– ions within the demineralized layers. The greater extent of mineralization observed in some crystals, especially those in the deeper layers, suggests more prominent remineralization. Xylitol formed strong complexes with Ca2+ and delayed the formation of precipitates of calcium phosphate. Xylitol seems to suppress the nucleation of the crystal. In addition, remineralization of the outermost surface layer might be suppressed, because increasing the fluidity of the xylitol solution is inappropriate for mineralization of the surface layer. This is the first study which explained the induction of remineralization in deep layers of demineralized enamel using a remineralizing solution containing xylitol.[21]
In the present study, Enafix (group A) had higher remineralization potential (226.37± 20.38 MPa) when compared to Gc tooth mousse plus and Clinpro tooth creme, but it was lower than Squigle (232.79 ± 41.04 MPa). Enafix (Casp) acts by adsorption of sucrose phosphate ions onto the enamel surface; thereby, it reduces the rate of acid dissolution of hydroxyapatite and enhances remineralization by calcium and phosphate ion by common ion effect. It quickly breaks down and releases calcium, phosphate, and sucrose phosphate ions into the saliva. These ions rapidly adsorb on the enamel surface; it inhibits demineralization and increases rapid remineralization. It can be concluded that periodic application of enafix toothpaste could significantly reduce the depth of enamel lesion produced subsequently by an acid challenge.
Casein phosphopeptide-amorphous calcium phosphate with fluoride (CPP ACPF) is a supersaturated solution of amorphous and crystalline calcium phosphate phases. As CPP– ACP can localize ACP at the tooth structure, increasing the level of calcium phosphate in plaque, and hence it acts as a calcium phosphate reservoir. It helps in buffering the free calcium and phosphate ion activities, thereby it helps to maintain a state of supersaturation for tooth enamel and decreases the enamel demineralization and enhances enamel remineralization.[4]
Fluoride, when added to CPP-ACP, gives a synergistic effect on remineralization of the early carious lesion Their mineralizing capacity is directly proportional to the levels of free calcium and phosphate ions that are stabilized by CPP. In this study Enafix showed higher SMH values than gc tooth mousse plus. George et al. evaluated the effect of calcium sucrose phosphate and calcium casein phosphopeptide containing pastes on mineralization of artificially demineralized human enamel. He concluded that Remineralization produced by calcium casein phosphopeptide is less when compared to calcium sucrose phosphate and is significant. With the high remineralizing ability of calcium sucrose phosphate, it can become the primary remineralizing agent in the treatment of early enamel caries.[22] It is suggested that CPP-ACP molecules need an acidic exposure to be activated and it should separate ACP from the casein. When it was necessary for activation they were washed by artificial saliva. This might be the reason for lower microhardness value of CPP-ACPFthan Casp and xylitol.[9], [10]
Clinpro tooth creme is a 0.21% w/w sodium fluoride (NaF) anticaries dentifrice that contains 950 ppm fluoride with functionalized tricalcium phosphate (f-TCP) as ingredient.[2] Tricalcium phosphate (TCP) ingredient is a new hybrid material created with a milling technique that fuses beta-tricalcium phosphate (ß-TCP) and sodium lauryl sulfate or fumaric acid. This blending results in "functionalized" calcium and a "free" phosphate, designed to increase the efficacy of fluoride remineralization. ß-TCP is similar to apatite structure and possesses unique calcium environments capable of reacting with fluoride and enamel. When the phosphate ions float freely, the exposed calcium environments are protected by preventing the calcium from prematurely interacting with fluoride.[23]
Clinpro tooth creme showed the lowest microhardness values because it forms complexes with calcium ions and preventing more general calcium phosphate precipitation.[24] Brar GS et al. stated that the synergistic anti-cariogenic effects are seen when CPP – ACP (GC Tooth Mousse Plus) and beta-tricalcium phosphate (β-TCP) are combined with fluoride (Clinpro tooth crème) are the same, but the reaction products formed by both are different. When the sample was viewed at six months, spherical globular agglomerates were observed in the reaction product layers produced by the GC tooth mousse plus application. Spherical agglomerates between the large surface clumps or mounds were evident. When compared with Clinpro Tooth Creme(β-TCP), the surface area covered by the spherical globules was more in GC tooth mousse plus group and the agglomerates of calcium fluoride globules formed on the enamel surface were denser. He concluded that GC tooth mousse plus were found to be active and better than Clinpro tooth creme.
Limitations of the Study
Long-term clinical trials should be carried out to prove the superiority of these materials in the vital teeth due to short period of application of remineralizing agents in this study.
Even though surface remineralization was confirmed, enamel subsurface remineralization was not evaluated in the study.
Conclusion
Within the limitations of the present study, it can be concluded that, Positive control group showed highest microhardness values among all the experimental groups, and the negative control showed the lowest values. Among the materials used, Xylitol (squigle) showed highest microhardness followed by calcium sucrose phosphate (Enafix), GC Tooth mousse plus and least in case of Sodium fluoride with TCP (Clinpro), but the difference is not statistically significant.
Source of Funding
None.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- EC Reynolds. Calcium phosphate‐based remineralization systems: scientific evidence. Aust Dent J 2008. [Google Scholar]
- A Balakrishnan, R Jonathan, P Benin, A Kuumar. Evaluation to determine the caries remineralization potential of three dentifrices: An in vitro study. J Conserv Dent 2013. [Google Scholar] [Crossref]
- DD Kalra, RD Kalra, PV Kini, CRA Prabhu. Nonfluoride remineralization: An evidence-based review of contemporary technologies. J Dent Allied Sci 2014. [Google Scholar] [Crossref]
- GK Divyapriya, PC Yavagal, DJ Veeresh. Casein phosphopeptide-amorphous calcium phosphate in dentistry: An update. Int J Oral Health Sci 2016. [Google Scholar] [Crossref]
- SR Joshi, GD Pendyala, MD Viddyasagar, N Padmawar, A Nara, P Joshi. Remineralizing agents in dentistry: A review. Int J Appl Dent Sci 2018. [Google Scholar]
- S. Sivaranjani, S Ahamed, Bhavani, Rajaraman. Comparative evaluation of remineralisation potential of three different dentifrices in artificially induced carious lesions: An invitro study. Int J Curr Res 2018. [Google Scholar]
- D Chole, Y Jadhav, S Kundoor, S Bakle, A Devagirkar, R Deshpande. Remineralizing Agents: Minimal Invasive Therapy A Review. IOSR J Dent Med Sci 2016. [Google Scholar]
- ME Reynolds. State Medicaid Rate Variations: Thanks For The Comparative Data. Health Affairs 1999. [Google Scholar] [Crossref]
- RL Karlinsey, AC Mackey, GK Stookey, AM Pfarrer. In vitro assessments of experimental NaF dentifrices containing a prospective calcium phosphate technology. Am J Dent 2009. [Google Scholar]
- C Hayes. The effect of non-cariogenic sweeteners on the prevention of dental caries: a review of the evidence. J Dent Educ 2001. [Google Scholar]
- AB Mehta, V Kumari, R Jose, V Izadikhah. Remineralization potential of bioactive glass and casein phosphopeptide-amorphous calcium phosphate on initial carious lesion: An in-vitro pH-cycling study. J Conserv Dent 2014. [Google Scholar] [Crossref]
- LM. Silverstone, CA Saxton, IL Dogon, O Fejerskov. Variation in the Pattern of Acid Etching of Human Dental Enamel Examined by Scanning Electron Microscopy. Caries Res 1975. [Google Scholar] [Crossref]
- DB Scott, JW Simmelink, V Nygaard. Structural Aspects of Dental Caries. J Dent Res 1974. [Google Scholar] [Crossref]
- LM Silverstone. Remineralization and enamel caries: new concepts. Dent Update 1983. [Google Scholar]
- S Huang, S Gao, L Cheng, H Yu. Combined effects of nano-hydroxyapatite and Galla chinensis on remineralisation of initial enamel lesion in vitro. J Dent 2010. [Google Scholar] [Crossref]
- M Assery, A Baothman. Effect of modified 5% sodium fluoride on the surface roughness and hardness of the enamel of primary incisors: An in vitro study. Saudi J Oral Sci 2017. [Google Scholar] [Crossref]
- DM Hamid. Effect of different remineralizing agents on the microhardness of therapeutic gamma irradiated human dentin. Dent J 2013. [Google Scholar]
- MP Gutiérrez-Salazar, J Reyes-Gasga. Microhardness and chemical composition of human tooth. Mater Res 2003. [Google Scholar] [Crossref]
- R Mittal, N Relhan, T Tangri. Remineralizing Agents: A Comprehensive Review. Int J Clin Prev Dent 2017. [Google Scholar] [Crossref]
- SS Shah. Xylitol: A Novel Remineralizing Agent: A Review. Int J Med Health Res 2017. [Google Scholar]
- Y Miake. Remineralization effects of xylitol on demineralized enamel. J Electron Microsc 2003. [Google Scholar] [Crossref]
- KJ George, F Rejula, JM Varughese, AS Babu, AS Gopinathan. Effect of calcium sucrose phosphate and calcium casein phosphopeptide containing pastes on mineralization of artificially demineralized human enamel an in vitro study. IOSR J Dent Med Sci 2019. [Google Scholar]
- NP Preethi. Remineralizing Agent -Then and Now -An Update. Dentistry 2014. [Google Scholar] [Crossref]
- C Zhou, D Zhang, Y Bai, S Li. Casein phosphopeptide-amorphous calcium phosphate remineralization of primary teeth early enamel lesions. J Dent 2014. [Google Scholar]
How to Cite This Article
Vancouver
Manduru CS, Chandra S, Moosani GK, Sampathi NR, Reddy U, Yalamatchi A. A comparative study of remineralizing efficiency of various remineralizing agents on artificially demineralized enamel surface – An in vitro study [Internet]. Int Dent J Stud Res. 2025 [cited 2025 Sep 09];9(2):94-100. Available from: https://doi.org/10.18231/j.idjsr.2021.018
APA
Manduru, C. S., Chandra, S., Moosani, G. K., Sampathi, N. R., Reddy, U., Yalamatchi, A. (2025). A comparative study of remineralizing efficiency of various remineralizing agents on artificially demineralized enamel surface – An in vitro study. Int Dent J Stud Res, 9(2), 94-100. https://doi.org/10.18231/j.idjsr.2021.018
MLA
Manduru, Chandra Sekhar, Chandra, Sushma, Moosani, Gopi Krishna, Sampathi, Nogalakshmi Reddy, Reddy, Upendranaha, Yalamatchi, Anusha. "A comparative study of remineralizing efficiency of various remineralizing agents on artificially demineralized enamel surface – An in vitro study." Int Dent J Stud Res, vol. 9, no. 2, 2025, pp. 94-100. https://doi.org/10.18231/j.idjsr.2021.018
Chicago
Manduru, C. S., Chandra, S., Moosani, G. K., Sampathi, N. R., Reddy, U., Yalamatchi, A.. "A comparative study of remineralizing efficiency of various remineralizing agents on artificially demineralized enamel surface – An in vitro study." Int Dent J Stud Res 9, no. 2 (2025): 94-100. https://doi.org/10.18231/j.idjsr.2021.018
