- Visibility 133 Views
- Downloads 35 Downloads
- Permissions
- DOI 10.18231/j.idjsr.2021.006
-
CrossMark
- Citation
Anti biofilm agents – Nature the cure
- Author Details:
-
Sheena S Raj *
-
Nebu George Thomas
Abstract
Microbiota are present in biofilms, which are highly structured and complex entities that are radically different from microbes in planktonic suspensions. Biofilms cause root canal infections. The root canal system's complexity and variability, combined with the multi-species presence of biofilms, make disinfection extremely difficult. The most critical reason for root canal treatment failure tends to be microbial persistence, which may have an effect on pain and quality of life.
In biomedical science, pathogenic microorganisms and their chronic pathogenicity are major concerns. Because of the prevalence of multidrug-resistant microbes in biofilms, chronic infections are difficult to treat. Researchers are searching for many effective natural anti-biofilm agents due to the low efficacy of various drugs and the in vivo toxicity of available antibiotics.
Rural extracts and natural product-based anti-biofilm agents are more effective and have less side effects than their chemically synthesised counterparts. The current review focuses on various natural anti-biofilm agents, such as phytochemicals, biosurfactants, antimicrobial peptides, and microbial enzymes, as well as their origins, mechanism of action through interfering in the quorum-sensing pathway, and mechanism of action via interfering in the quorum-sensing pathway.
Introduction
A biofilm is a highly ordered structure made up of bacteria enclosed in an extracellular polymeric matrix that is attached to a surface. Biofilms may also be thought of as a layer of microbiota condensation or a microbial derived culture made up of cells that are irreversibly bound to a substrate or interface, as well as to each other, and embedded in an extracellular matrix.[1], [2] The concept of biofilm was first developed by Marshall et al. (1971) and further described by Fletcher, Characklis, and Costerton describes Biofilm as a sessile multicellular microbial community characterised by cells that are firmly attached to a surface and enmeshed in a self- produced matrix of extracellular polymeric substance (EPS), usually a polysaccharide.[3] Free-floating bacterial cells vary greatly from sessile bacterial cells (biofilm state) (planktonic state). Bacteria in biofilms have different physiological properties than bacteria in culture media, partially because microorganisms in biofilms are shielded from environmental stresses by their matrix.[4] The precise composition varies depending on the microorganisms and nutrients available. The organisms in the biofilms exhibit an altered phenotype with respect to growth rate and gene transcription
The root canal system however, is highly complex with isthmuses, lateral extensions, apical deltas, lateral canals and dentinal tubules, [5], [6] providing shelter for the microorganisms against the action of instruments and disinfectants. In addition, the biofilm lifestyle of the bacteria in the root canal poses additional challenges. The microbial cells are attached to the canal walls, and they are embedded within a self‐produced extracellular matrix. Compared to their planktonic counterparts, cells in a biofilm are much more tolerant to most antimicrobials and the host defence.[7] Reduced penetration of antimicrobial agents through the biofilm matrix, biofilm‐specific protection against oxidative stress and biofilm‐specific expression of efflux pumps are some mechanisms that explain the reduced susceptibility of biofilm cells.[8] Also, the endodontic biofilm is more or less continuous throughout the anatomical irregularities of the canal system, which imposes significant challenges to effective debridement and disinfection.[9]
This review aims to focus on the natural anti-biofilm agents effective against a broad range of microbial biofilms and strategies related to recent biofilm treatments.
Anti-biofilm Agents Based on Natural Products
The formation and growth of biofilm is a complex process with multiple stages that can be targeted by natural anti-biofilm agents to inhibit biofilm formation. The stages of biofilm development include (1) attachment of bacterial cells to a suitable biotic/abiotic surface, (2) development of biofilm structure, (3) maturation of biofilm and (4) dispersion (Boles and Horswill, 2008).[10] The first two stages are extremely important in the formation of biofilms and inhibit one or both of them appear to be the best strategy for preventing biofilm formation. Key players in the attachment stage are cytoskeletal elements (most notably flagella and fimbriae) and lipopolysaccharides. The formation of biofilm is aided by the surface signaling/communication of a group of bacteria, also known as Quorum Sensing. The natural anti-biofilm agents either acts solely or synergistically by diverse mechanisms.
Phytochemicals
There are broadly five classes of natural compounds that have high anti-biofilm properties. Those are phenolics, essential oils, terpenoids, lectins, alkaloids, polypeptides and polyacetylenes (Yong et al., 2019).[11] Phenolics are a class of chemicals. Phenolic acids, quinones, flavonoids, flavones, flavonols, tannins, and coumarins are among the seven subclasses, with tannins, particularly condensed tannins, having anti-biofilm activity (Trentin et al., 2011).[12] These compounds act on biofilm through six major mechanisms, including substrate deprivation, membrane disruption, and binding to adhesin complexes and cell walls, as well as binding to proteins, interacting with eukaryotic DNA, and blocking viral fusion (Cowan, 1999; Lu et al., 2019).[13], [14] Several solvents were used to extract natural compounds from different sources for anti-biofilm activity, including water, methanol, ethanol, chloroform, ether, dichloromethanol, and acetone. Researchers discovered that water extracts anthocyanins, sugars like tannins, saponins, terpenoids, polypeptides, and lectins in a variety of experiments. Methanol extracts anthocyanins, terpenoids, saponins, tannins, xanthoxyllines, quassinoids, totarol, flavones, lactones, phenones, and polyphenols, while ethanol extracts tannins, polyphenols, polyacetylenes, flavonol, terpenoids, sterols, alkaloids, and propolis (Cowan 1999).[14] Extraction with chloroform yields terpenoids and flavonoids; dichloromethanol yields only terpenoids ethers when used as solvent results in the extraction of terpenoids, alkaloids, fatty acids, and coumarins whereas acetone isolates flavonols. Hydroquinone and caffeic acid methyl ester, isolated from Cnestis ferruginea Vahl ex DC. aqueous extract, showed promising results against S. aureus (Kouakou et al., 2019).[15] In vitro tests showed that methoxy-trans-carnosic acid and carnosol isolated from the methanolic extract of Salvia officinalis L., an Algerian medicinal plant, had anti-biofilm activity against Candida biofilm (Kerkoub et al., 2018). [16]
Phytochemicals work by blocking quorum sensing inducers like AHL, autoinducers, and autoinducers type 2 to disrupt the quorum sensing mechanism (Ciric et al., 2019).[17] Garlic extracts are important in inhibiting quorum sensing signaling molecules in biofilms of Pseudomonas and Vibrio spp (Harjai et al., 2010). [18] According to many experts, quorum quenchers, along with antibiotics, are the best alternative anti-biofilm agents (Paluch et al., 2020).[19] Phytochemicals also play a role in bacterial adhesion inhibition and the suppression of genes involved in biofilm formation (Adnan et al., 2020). [20] Interfering with the forces (Van der Waals force of attraction, electrostatic attraction, sedimentation, and Brownian movements) that support bacterial attachment to different surfaces may prevent biofilm growth in its early stages (Roy et al., 2018).[21] Phytocompounds have the ability to prevent access to nutrients required for adhesion and bacterial growth, as well as to interfere with the extension process (Sandasi et al., 2010).[22]
Biosurfactants
Biosurfactants (BS) prevent biofilm formation by altering cell adhesion ability through reduced cell surface hydrophobicity, membrane disruption, and inhibition of the electron transport chain, lowering cellular energy demands (Satpute et al., 2016). [23] Various microorganisms make biosurfactants of various classes that have antibacterial, antifungal, and anti-biofilm properties (Paraszkiewicz et al., 2019).[24] The impact of biosurfactants from Lactobacillus plantarum and Pediococcus acidilactici on quorum sensing signaling molecules and biofilm-related gene expression in Staphylococcus aureus was studied (Yan et al., 2019).[25] At 50 mg/ml, Pediococcus acidilactici biosurfactant inhibits autoinducer-2 (AI-2) signaling molecules, accessory gene regulator (agr A), and staphylococcal accessory regulatory (sar A) gene expression (Yan et al., 2019).[25] Previous research found that Lactobacillus-derived BS loaded liposomes were more effective than free BS at inhibiting S. aureus (MRSA) biofilm formation and elimination (Giordani et al., 2019).[26] An anionic lipopeptide from Acinetobacter junii that self-aggregates to form sheet–rich biosurfactant vesicles was discovered by Ohadi et al. (2020). This biosurfactant can be used as an anti-biofilm agent because it is thermostable and less toxic. Dermatophytes produce biofilms that are extremely difficult to remove. In ex vivo conditions for M. canis, a lipopeptide biosurfactant derived from Beauveria bassiana, an insect-attacking fungus, plays an important role as an anti-biofilm agent (Abdel-Aziz et al., 2020).[27] It works by interfering with cell membrane permeability and disrupting cell membrane integrity. Since it was made from steep corn liquor, the biosurfactant from B. bassiana overcame the disadvantage of being expensive to make. For recalcitrant dermatophytosis, this could be a promising biosurfactant. Surfactin, a cyclic lipopeptide, was found to be very effective against C. albicans biofilm-related infections when combined with its metal complex. This biosurfactant also regulates the expression of hyphal-specific genes, primarily by lowering the hydrophobicity of the cellular surface (Janek et al., 2020).[28] Biosurfactants are suitable coating agents for medical implants such as urinal catheters, bone implants, and so on, to prevent pathogenic organisms from forming biofilms without the use of synthetic drugs. Rhamnolipids and sorphorolipids have been identified as potential inhibitors of Gram-negative and Gram-positive microbe biofilm formation (Sharahi et al., 2019).[29] Proteus vulgaris and Staphylococcus aureus biofilm formation on polydimethylsiloxane (PDMS)-based implants are inhibited by cell-associated biosurfactant from Lactobacillus acidophilus, according to a few studies (Satpute et al., 2019). [30]
Antimicrobial Peptides (AMPs)
AMPs are antimicrobials with a broad spectrum of action that are commonly used to treat both fungal and bacterial biofilms (Pletzer et al., 2016).[31] These peptides break up biofilms on medical devices like catheters, artificial valves, stents, and dentures that are used in hospital-acquired infections by S. aureus, Klebsiella pneumoniae, P. aeruginosa, Enterococcus faecium, Acinetobacter, and Enterobacter spp. (ESKAPE), as well as non-ESKAPE pathogens (Rajput and Kumar, 2018).[32] AMPs are a type of antibiotic that attacks the bacterial cell membrane, making them less susceptible to bacterial resistance (Hirt et al., 2018).[33] AMPs are found in humans, animals, plants, and microbes and act on bacterial cell membranes by electrostatically interacting with membrane phospholipids, then insertion into the membrane, killing bacteria. Synergizing AMPs with antimicrobial compounds has been reported to inhibit various molecular pathways involved in biofilm formation (Shahrour et al., 2019).[34]
Many AMPs found in amphibian skin are effective against biofilm-causing microorganisms. Yuan et al. (2019) isolated an AMP Japonicin-2LF from the skin secretion of a Fujian large-headed frog (Limnonectes fujianensis) that prevents MRSA biofilms by permeabilizing the membrane. Japonicin-2LF acts as a detergent in biofilms, killing both planktonic and sessile bacteria. This property can be used to develop this peptide as a promising drug candidate for the treatment of MRSA infection in cystic fibrosis patients. The major disadvantage of using AMPs to treat biofilm-based infections is that they are highly susceptible to bacterial protease degradation.
Therapeutic Strategies Using Natural Products
Because traditional antibiotic therapies have failed, biofilm treatments will need to be upgraded (Zhang et al., 2020). [35] Natural anti-biofilm agents selectively kill persistent biofilms while allowing antimicrobials to diffuse into the biofilm matrix. These natural products work to degrade the biofilm matrix and destroy the released cells at various stages of the biofilm cycle ([Figure 1]). Researchers will be able to design better anti-biofilm strategies if they have a better understanding of how biofilms interfere and disperse.
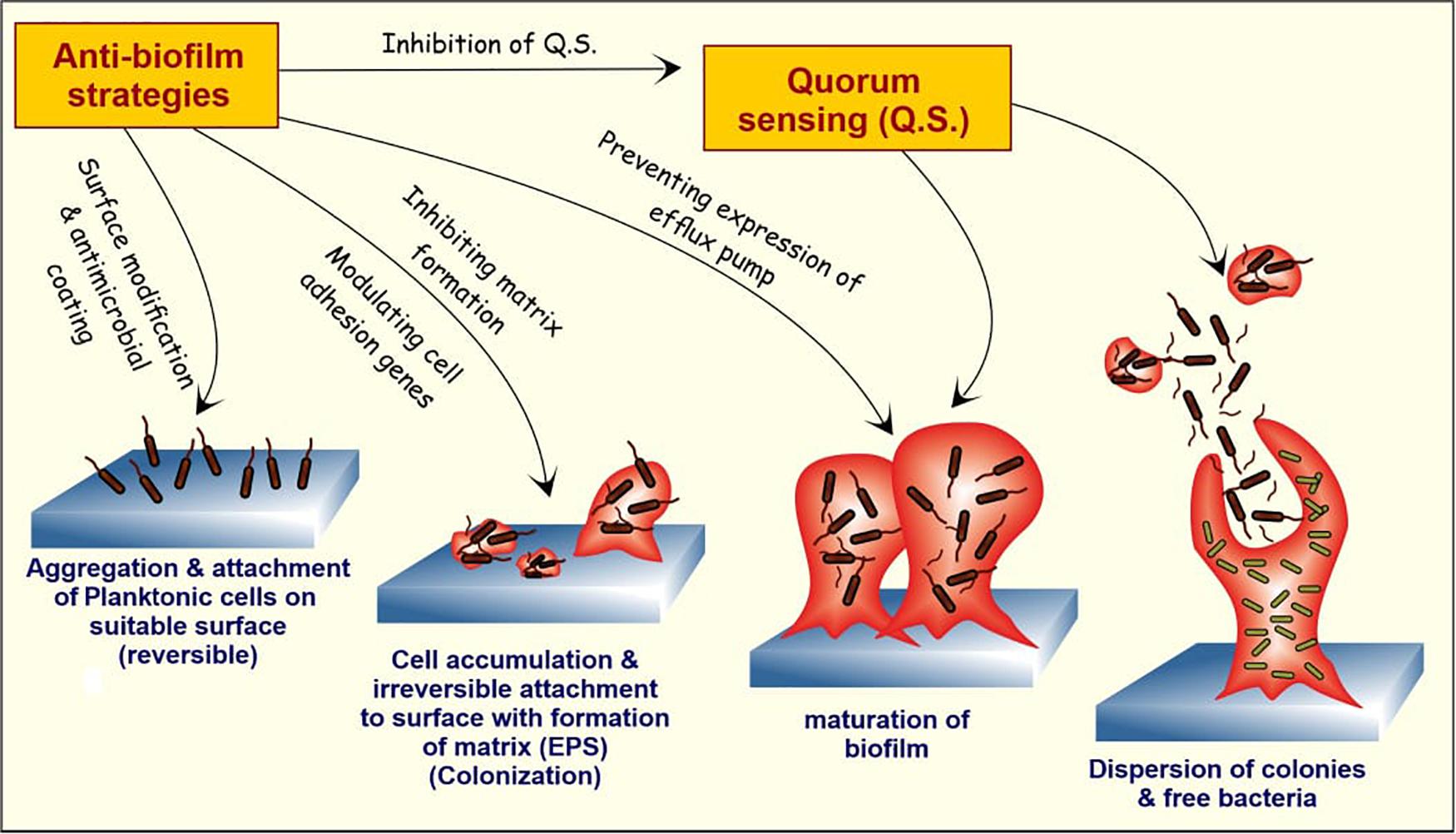
Elasnin (an anti-biofilm compound derived from the actinobacteria Streptomyces mobaraensis DSM 40847) was found to kill the matrix in a multispecies biofilm, making it more susceptible to antibiotics, according to a recent study (Long et al., 2020). [36] The aim of this review is to use natural agents to create an efficient and safe biofilm inhibition strategy, which will help to improve current biofilm inhibition strategies. It goes over some of the existing technologies in use to disintegrate EPS, quench QS networks, prevent adhesion, and stop biofilm formation. ([Figure 2] )
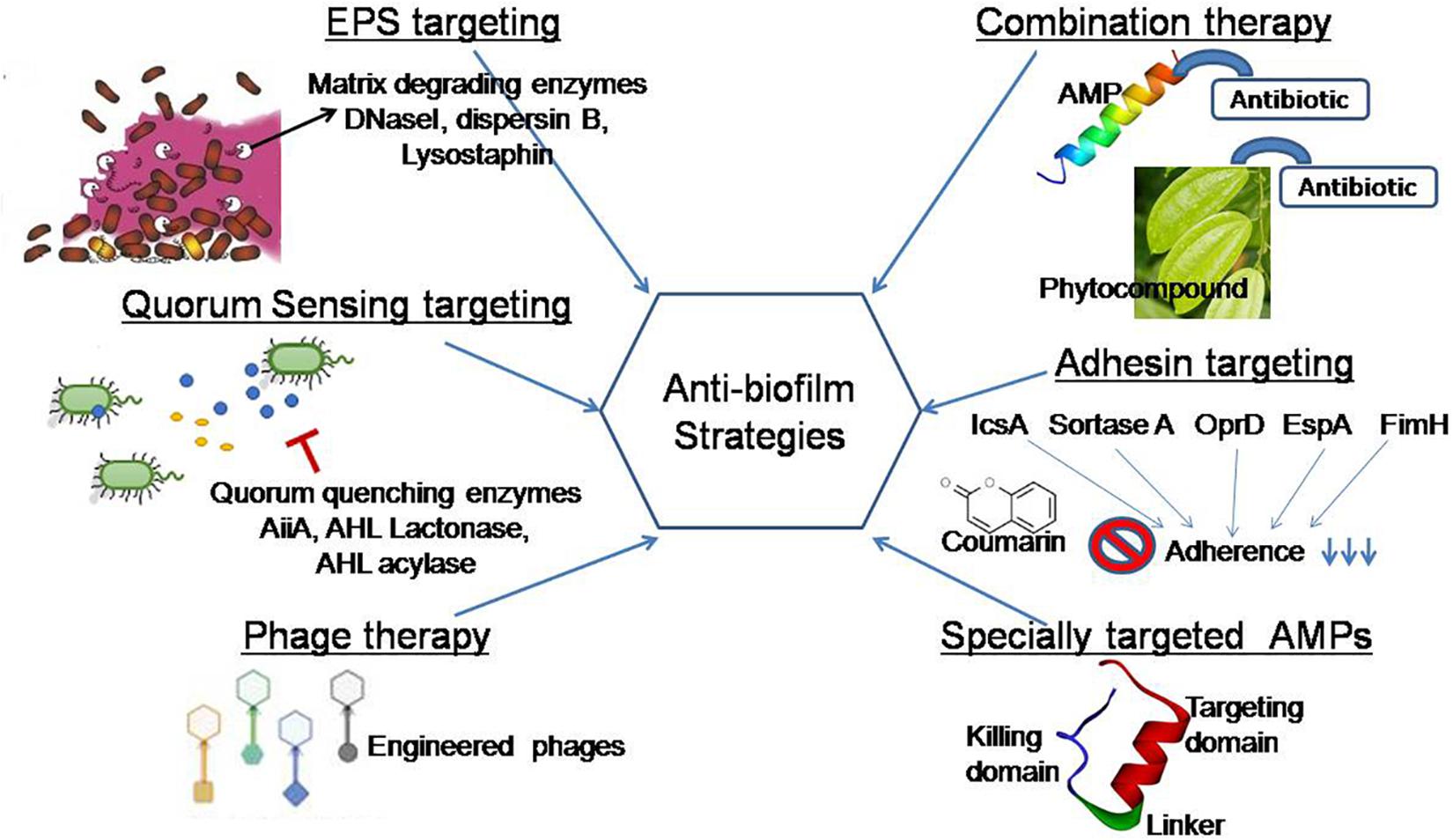
Polysaccharides, structural proteins, and extracellular DNA are the primary components of microbial EPSs are secreted by a wide range of microorganisms. The EPS matrix promotes microbial adhesion to surfaces, multilayered biofilm aggregation, and serves as a three-dimensional scaffold for hydration, digestion, and resistance to antimicrobial compounds, antibiotics, and host effector molecules (Flemming et al., 2016a).[1] The EPS matrix has the ability to actively change nutrient gradients and depict pathogenic environments that influence tolerance and virulence characteristics. As a result, many therapeutic strategies aim to eliminate biofilms, disaggregate bacteria, and disrupt the pathogenic environment by targeting the EPS matrix. Many bacterial enzymes and secondary metabolites interfere with pathogenic bacteria's quorum sensing mechanisms, disrupting biofilm formation (Khan et al., 2019). [37] The Gram-negative periodontal pathogen Actinobacillus actinomycetemcomitans secretes biofilm matrix-degrading enzymes like beta-N-acetylglucosaminidase and dispersin B, which disintegrate mature Staphylococcus epidermidis biofilms. In an in vivo pig skin colonization model, a cocktail of two EPS-degrading enzymes, DNase I and dispersin B, was found to inhibit staphylococcal skin colonization, remove pre-attached S. aureus cells from the skin, and increase their povidone-iodine susceptibility (Kaplan et al., 2018). [38] According to Hogan et al. (2017), lysostaphin is an effective anti-staphylococcal agent that can be improved when used in conjunction with antibiotics.
By using a modeling and engineering approach, existing enzymes with low catalytic activity can improve their catalytic properties against biofilms. Another approach to modulating the enzymes' biofilm-inhibitory properties is to use site-directed mutational analysis. As a result, broad-spectrum enzymes/peptides, as well as secondary compounds, must be isolated from bacteria for bioprospecting, as they can target a wide range of QS signaling molecules and biofilm structural components. Hydrolytic enzymes that can degrade proteins, polysaccharides, eDNA, and QS molecules must be combined to completely eliminate heterogeneous biofilms (Yuan et al., 2020). [39] Due to cost, handling procedures, and low industrial accessibility, the use of matrix-degrading enzymes in biofilm control is currently limited (Nahar et al., 2018). [40]
Quorum Sensing Targeting Strategies
Quorum sensing (the prevention of cell-to-cell communication) is an effective approach for preventing biofilm formation (Sharahi et al., 2019). [41] It has been discovered that the metalloprotein AHL-lactonase found in the cell-free extract of endophytic Enterobacter species degrades N-AHL, preventing Aeromonas hydrophila from forming biofilms (Shastry et al., 2019).42 Lactobacillus crustorum ZHG 2-1, as novel quorum-quenching bacteria, degrades N-3-oxodecanoyl-dl-homoserine lactone (3-oxo-C12-HSL) and N-butyryl-dl-homoserine lactone (C4-HSL) and acts as an anti-biofilm agent against P. aeruginosa, according to a recent study (Cui et al., 2020). [42] There have been many reports of quorum quenching (QQ) enzymes and compounds. The vast majority of these QQ molecules came from natural sources (LaSarre and Federle, 2013). [43] A recent study found that ethyl acetate extracts from cell-free supernatants and cells of the Natrinemaversi forma have QS inhibitory potential against P. aeruginosa biofilm (Başaran et al., 2020).[44] Many QS inhibitors derived from plant-based natural products have been identified and suggested to be effective in future biofilm targeting strategies (Caceres et al., 2020; Zhong et al., 2020).[45]
Quorum quenchers, on the other hand, are generally species specific; thus, to remove mixed-species biofilms, a combination of quenchers is needed. In both Gram-negative (P. aeruginosa) and Gram-positive (S. aureus) bacteria, ajoene, a sulfur-rich molecule found in garlic, decreases the expression of small regulatory RNAs (sRNAs). Ajoene is the first compound to target broad-spectrum quorum sensing inhibitors, reducing RNAIII expressions in S. aureus (Scoffone et al., 2019)[46] and RsmY and RsmZ expressions in P. aeruginosa (Scoffone et al., 2019). (Jakobsen et al., 2017).[47]
By downregulating genes in the QS system, the anti-biofilm peptide Human Cathelicidin LL-37 affects the bacterial cell signaling system and prevents P. aeruginosa biofilm formation at 0.5 g/ml (Di Somma et al., 2020).[48] AMPs interact with bacteria's membranes, causing them to activate genes controlled by QS. Membrane vesicles helped these QS autoinducers pass through the plasma membrane. The expression of virulence genes linked to QS is then activated as a result of this process. Small autoinducing peptide molecule (AIP) from Lactobacilli inhibits the viability of microbes and acts as a suppressor of bacteriotoxin production; one intriguing autoinducer is small autoinducing peptide molecule (AIP) from Lactobacilli that inhibits the viability of microbes and acts as a suppressor of bacteriotoxin production. They interfere with the agr QS system during the suppression of exotoxin production (Vasilchenko and Rogozhin, 2019). However, since quorum quenchers can be washed away during biofilm formation, these inhibitors are only used in small areas of biofilm (Koo et al., 2017). [49] As a result, combining these inhibitors with other techniques results in a novel therapeutic strategy.
Phage Therapy
Lytic bacteriophages have been shown to be an effective treatment for removing biofilm cells. Two lytic phages, vB SauM ME18 and vB SauM ME126, were recently discovered to be potential natural antimicrobials for inhibiting MDR S. aureus biofilm (Gharieb et al., 2020). [50] Recent research has shown that (engineered) phage-derived enzymes, such as polysaccharide depolymerase or peptidoglycan-degrading enzymes, are promising anti-biofilm therapeutic candidates (Reuter and Kruger, 2020). [51] Patients underwent phage treatment at the School of Medicine, University of California San Diego (UCSD) phage therapy center, which obtained its first FDA approval in 2019. Phage therapy is only used in a few countries, and it faces numerous challenges in clinical practice, including the establishment of phage banks with well-characterized phages; the safety, stability, and quality of phage preparations during production; and the evolution of bacterial resistance to phages.
Combination Therapy
Antibiotics are sensitized by natural anti-biofilm agents, which have been shown to be more effective when used in amalgamation (Zhang et al., 2020). [52] They also discovered that using sodium houttuyfonate and levofloxacin together inhibits biofilm formation more effectively. P. aeruginosa biofilm dispersion is successfully disrupted by sodium houttuyfonate, a plant-derived anti-neuropeptide (Wang et al., 2019).[53] In contrast to individual therapy with the marketed antibiotics ciprofloxacin and tetracycline, naringin, a flavanone glycoside extracted from citrus and grapefruits, was found to be more effective against P. aeruginosa biofilms (Dey et al., 2020). [54] Naringin inhibits pellicle formation and decreases the flagellar movement of bacteria on catheter surfaces by depleting biofilm EPS and facilitating antimicrobial diffusion.
Zhou et al. (2018) investigated the effects of hordenine, a polyphenolic compound derived from barley, on biofilm formation alone and in combination with netilmicin, an aminoglycoside antibiotic. The results were promising, with a combination of hordenine and netilmicin reducing P. aeruginosa PAO1 biofilms by up to 88 percent, which was significantly better than any of the individual therapies. It suggests that drug–herb combination therapy should be investigated for anti-biofilm formulation possibilities. The biofilm layer's thickness was reduced, and its architecture was disrupted, according to the SEM analysis. Actinobacterial compounds from various microbial species have also demonstrated potential anti-biofilm activity against pathogenic bacteria by disrupting the cell surface and cell-cell interaction (Azman et al., 2019). [55] Studies combining multiple natural anti-biofilm compound/s from various sources or acting on different stages of biofilm development would aid in the development of more effective biofilm-targeting agents. Furthermore, choosing a more effective compound is necessary because natural compounds' effectiveness against biofilm growth varies depending on the bacteria strain.
Anti-biofilm Biomaterial Therapy
Because biofilm-associated pathogenic organisms' adhesion to implant surfaces limits their clinical utility, various researchers have attempted to coat biomaterial as a preventive strategy. Anti-adhesive coatings of algal polysaccharide ulvan, dextran, and dermatan sulfate, as well as antimicrobial-releasing polysaccharide coatings, have become increasingly prevalent over the last decade (Junter et al., 2016). [56] The hydrophilic polysaccharides form a hydration layer on the surface, which acts as a physical barrier and inhibits cell adhesion to the surface (Damodaran and Murthy, 2016). [57] Calcium phosphate cement and hydroxyapatite are calcium phosphate materials used as a bone coating to prevent biofilm infections, but clinical trials have shown that they have a number of drawbacks (Pan et al., 2018). Chitosan hydrogel coatings, which inhibit bacterial adhesion and biofilm formation due to membrane leaching, can help prevent implant-related infections (Pan et al., 2018). [58] Fibers, strips, gels (Badam gum, Karaya gum, chitosan), films (chitosan), nanoparticles, and microparticles were used as drug transporters in various forms to help deliver antibiotics to the targeted site, primarily for periodontal biofilm-forming pathogens (Chi et al., 2019). [59] Nisin, an FDA-approved AMP, fights methicillin-resistant Staphylococcus aureus, Streptococcus pneumoniae, Enterococci, and Clostridium difficile by acting as an anti-biofilm agent in combination with traditional antibiotics (Shin et al., 2016). [60] According to a recent report, nisin in combination with gellan gum, a biocompatible polysaccharide, has shown promise in biomaterial studies (Peng et al., 2020). [61]
Conclusion and Future Directions
In medicine and human health, the occurrence of many biofilm-based human infections and their multiple antimicrobial resistance is a major concern. The discovery and characterization of novel natural anti-biofilm agents is prompted by the increased rate of antibiotic resistance in biofilm. This review discusses various phytocompounds, antimicrobial peptides, and biosurfactants that have shown to inhibit biofilm formation. Natural anti-biofilm agents may be useful in some surgeries and illnesses where untraceable infection sites, such as bone, dental, eye lenses, and breast implants are a possibility. In contrast to traditional antibiotics, these natural agents are more structurally and functionally diverse. Natural anti-biofilm agents from a variety of sources have been used to create a number of advanced therapeutic strategies with improved activity, stability, and reliability. We continue to investigate the effectiveness of specifically targeted AMPs against drug-tolerant pathogenic biofilms without disrupting the natural microflora in this paper. Natural products, primarily phytochemicals, have been investigated more in vitro and in vivo as anti-biofilm agents, but despite extensive research, no FDA-approved drug has been produced. In phase II and phase III clinical trials, the majority of them failed (Lu et al., 2019). [62] The availability of the compound in humans after administration, which reduces the effectiveness of the compounds, may be the cause of this failure. For better results, a combination of strategies such as antibiotics and natural anti-biofilm agents could be used to solve this problem. In order to advance anti-biofilm activity, future research should focus on combining natural agents with commercial antibiotics. Natural-source quorum quenchers combined with antibiotics could be a novel lead for species-specific biofilm destruction, with potential applications in biomedical industries. More research into converting novel anti-biofilm phytocompounds into drugs should be conducted. The majority of clinical trials on natural anti-biofilm compounds listed on http://clinicaltrials.gov/ are for oral biofilms, with only a few for urinary tract infections (Lu et al., 2019). In the future, more in vivo studies and clinical trials will be required to assess the efficacy of natural anti-biofilm agents.
The review also discusses natural quorum quenching molecules and EPS-degrading enzymes, as well as their mode of action on different biofilms. Various natural agents' mechanisms of action against biofilm are unknown. More research into the mode of action may aid in the discovery of new anti-biofilm agents. Because it targets and inhibits bacteria from adhering to the cell surface, the anti-adhesin strategy can be a novel approach for biofilm therapies on a wide range of bacteria. Since there have been few studies in this area, future research focusing on biofilm targeting adhesin proteins may lead to the discovery of novel natural anti-biofilm agents. Biofilm formation can be regulated by pili and curli gene expression controlling phytocompounds. More research in this area, or a combination of phytocompounds with anti-adhesin properties, may be a better therapeutic strategy for biofilm-related illnesses. Rigid quality control should prevent natural medicines from failing in clinical trials. The development of precise, sensitive, and stable markers will help solve the problem and improve the quality control of natural anti-biofilm agents. Natural product research faces a great challenge in finding useful QC markers because natural compounds have a very complex structural lattice (Zhang et al., 2020). Natural compound drug efficacy is primarily determined using network pharmacology techniques. As a result, more studies in this area could improve the final success rate of clinical trials. Novel natural anti-biofilm agents in therapeutics may be feasible if comprehensive studies in quality control, pharmacokinetic and pharmacodynamic co-relationships (PK–PD), and PK–PD interactions with metabolomics of the host are carried out for the assessment of the drug's safety and effectiveness.
Conflicts of Interest
All contributing authors declare no conflicts of interest.
Source of Funding
None.
References
- Flemming H, Wingender J, Szewzyk U, Steinberg P, Rice S, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563-75. [Google Scholar] [Crossref]
- Marsh PD. Dental plaque as a microbial biofilm. Caries Res. 2004;38(3):204-11. [Google Scholar]
- Mishra R, Panda A, Mandal S, Shakeel M, Bisht S, Khan J. Natural Anti-biofilm Agents: Strategies to Control Biofilm-Forming Pathogens. Front Microbiol. 2020;11. [Google Scholar] [Crossref]
- Caggianiello G, Kleerebezem M, Spano G. Exopolysaccharides produced by lactic acid bacteria: from health-promoting benefits to stress tolerance mechanisms. Appl Microbiol Biotechnol. 2016;100:3877-86. [Google Scholar] [Crossref]
- Versiani M, Pécora J, Sousa-Neto Md. Root and Root Canal Morphology of Four-rooted Maxillary Second Molars: A Micro–Computed Tomography Study. J Endod. 2012;38(7):977-82. [Google Scholar] [Crossref]
- Vertucci F. Root canal anatomy of the human permanent teeth. Oral Surg, Oral Med, Oral Pathol. 1984;58:589-99. [Google Scholar] [Crossref]
- Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS. 2013;121:1-58. [Google Scholar] [Crossref]
- Acker H, Dijck P, Coenye T. Molecular mechanisms of antimicrobial tolerance and resistance in bacterial and fungal biofilms. Trends Microbiol. 2014;22(6):326-33. [Google Scholar] [Crossref]
- Nair PR. Light and electron microscopic studies of root canal flora and periapical lesions. J Endod. 1987;13(1):29-39. [Google Scholar]
- Boles B, Horswill A. agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008;4(4). [Google Scholar] [Crossref]
- Yong Y, Dykes G, Choo W. Biofilm formation by staphylococci in health-related environments and recent reports on their control using natural compounds. Crit Rev Microbiol. 2019;45(2):201-22. [Google Scholar] [Crossref]
- DT, RG, KZ, AS, MS, MC. Potential of medicinal plants from the Brazilian semi-arid region (Caatinga) against Staphylococcus epidermidis planktonic and biofilm lifestyles. J Ethnopharmacol. 2011;137(1):327-35. [Google Scholar] [Crossref]
- Cowan MM. Plant products as antimicrobial agents.. Clin Microbiol Rev. 1999;12:564-82. [Google Scholar]
- Lu L, Hu W, Tian Z, Yuan D, Yi G, Zhou Y. Developing natural products as potential anti-biofilm agents. Chin Med. 2019;14:1-7. [Google Scholar] [Crossref]
- Kouakou K, Panda S, Yang M, Lu J, Jiang Z, Puyvelde L. Isolation of Antimicrobial Compounds From Cnestis ferruginea Vahl ex. DC (Connaraceae) Leaves Through Bioassay-Guided Fractionation. Front Microbiol. 2019;10. [Google Scholar] [Crossref]
- Kerkoub N, Panda S, Yang M, Lu J, Jiang Z, Nasri H. Bioassay-Guided Isolation of Anti-Candida Biofilm Compounds From Methanol Extracts of the Aerial Parts of Salvia officinalis (Annaba, Algeria). Front Pharmacol. 2018;9. [Google Scholar] [Crossref]
- Ćirić A, Petrović J, Glamočlija J, Smiljković M, Nikolić M, Stojković D. Natural products as biofilm formation antagonists and regulators of quorum sensing functions: A comprehensive review update and future trends. South Afr J Botany. 2019;120:65-80. [Google Scholar] [Crossref]
- Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence ofPseudomonas aeruginosa. FEMS Immunol Med Microbiol. 2010;58(2):161-8. [Google Scholar] [Crossref]
- Paluch E, Rewak-Soroczyńska J, Jędrusik I, Mazurkiewicz E, Jermakow K. Prevention of biofilm formation by quorum quenching. Appl Microbiol Biotechnol. 2020;104:1871-81. [Google Scholar] [Crossref]
- Adnan M, Patel M, Deshpande S, Alreshidi M, Siddiqui A, Reddy M. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion With Its Antibacterial Activities Against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front Microbiol. 2020. [Google Scholar] [Crossref]
- Roy R, Tiwari M, Donelli G, Tiwari V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9:522-54. [Google Scholar] [Crossref]
- Sandasi M, Leonard C, Viljoen A. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol. 2010;50(1):30-5. [Google Scholar] [Crossref]
- Satputea S, Banpurkar A, Banat I, Sangshetti J, Patil R, Gade W. Multiple Roles of Biosurfactants in Biofilms. Curr Pharm Des. 2016;22:1429-48. [Google Scholar] [Crossref]
- Satpute K, Kalyankar T. Investigation of Antiacne activity of some Medicinal Plants against Pathogenic Bacteria. Res J Pharm Technol. 2018;11. [Google Scholar] [Crossref]
- Yan X, Gu S, Cui X, Shi Y, Wen S, Chen H. ntimicrobial, anti-adhesive and anti-biofilm potential of biosurfactants isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microbial Pathogenesis. 2019;127:12-20. [Google Scholar]
- Giordani B, Costantini P, Fedi S, Cappelletti M, Abruzzo A, Parolin C. Liposomes containing biosurfactants isolated from Lactobacillus gasseri exert antibiofilm activity against methicillin resistant Staphylococcus aureus strains. Eur J Pharm Biopharmaceutics. 2019;139:246-52. [Google Scholar]
- Abdel-Aziz M, Al-Omar M, Mohammed H, Emam T. In Vitro and Ex Vivo Antibiofilm Activity of a Lipopeptide Biosurfactant Produced by the Entomopathogenic Beauveria bassiana Strain against Microsporum canis. Microorganisms. 2020;8. [Google Scholar] [Crossref]
- Janek T, Drzymała K, Dobrowolski A. In vitro efficacy of the lipopeptide biosurfactant surfactin-C15 and its complexes with divalent counterions to inhibit Candida albicans biofilm and hyphal formation. Biofouling. 2020;36(2):210-21. [Google Scholar] [Crossref]
- Sharahi J, Azimi T, Shariati A, Safari H, Tehrani M, Hashemi A. Advanced strategies for combating bacterial biofilms. J Cell Physiol. 2019;234(9):14689-708. [Google Scholar] [Crossref]
- Satpute S, Mone N, Das P, Banat I, Banpurkar A. Inhibition of pathogenic bacterial biofilms on PDMS based implants by L. acidophilus derived biosurfactant. BMC Microbiol. 2019;19(1):1-5. [Google Scholar] [Crossref]
- Pletzer D, Coleman S, Hancock R. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr Opin Microbiol. 2016;33:35-40. [Google Scholar] [Crossref]
- Rajput A, Kumar M. Anti-biofilm peptides: a new class of quorum quenchers and their prospective therapeutic applications. InBiotechnological Applications of Quorum Sensing Inhibitors. . 2018. [Google Scholar]
- Hirt H, Hall J, Larson E, Gorr S. A D-enantiomer of the antimicrobial peptide GL13K evades antimicrobial resistance in the Gram positive bacteria Enterococcus faecalis and Streptococcus gordonii. PLOS ONE. 2018;13(3). [Google Scholar] [Crossref]
- Shahrour H, Ferrer-Espada R, Dandache I, Bárcena-Varela S, Sánchez-Gómez S, Chokr A. AMPs as anti-biofilm agents for human therapy and prophylaxis. Antimicrob Pept. ;2019:257-79. [Google Scholar]
- Zhang L, Liang E, Cheng Y, Mahmood T, Ge F, Zhou K. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection?. Biomed Pharmacother. 2020;128. [Google Scholar] [Crossref]
- Long L, Chiang HY, Qian PY. A potent antibiofilm agent inhibits and eradicates mono-and multi-species biofilms. bioRxiv. 2020. [Google Scholar]
- Khan F, Oloketuyi S, Kim Y. Diversity of Bacteria and Bacterial Products as Antibiofilm and Antiquorum Sensing Drugs Against Pathogenic Bacteria. Current Drug Targets. 2019;20(11):1156-79. [Google Scholar] [Crossref]
- Kaplan J, Mlynek K, Hettiarachchi H, Alamneh Y, Biggemann L, Zurawski D. Extracellular polymeric substance (EPS)-degrading enzymes reduce staphylococcal surface attachment and biocide resistance on pig skin in vivo. PLOS ONE. 2018;13(10). [Google Scholar] [Crossref]
- Yuan L, Hansen M, Røder H, Wang N, Burmølle M, He G. Mixed-species biofilms in the food industry: Current knowledge and novel control strategies. Crit Rev Food Sci Nutr. 2020;60(13):2277-93. [Google Scholar] [Crossref]
- Nahar S, Mizan M, Ha AJ, Ha S. Advances and Future Prospects of Enzyme-Based Biofilm Prevention Approaches in the Food Industry. Comprehensive Rev Food Sci Food Safety. 2018;17(6):1484-502. [Google Scholar] [Crossref]
- Sharahi J, Azimi T, Shariati A, Safari H, Tehrani M, Hashemi A. Advanced strategies for combating bacterial biofilms. J Cell Physiol. 2019;234(9):14689-708. [Google Scholar] [Crossref]
- Cui T, Bai F, Sun M, Lv X, Li X, Zhang D. Lactobacillus crustorum ZHG 2-1 as novel quorum-quenching bacteria reducing virulence factors and biofilms formation of Pseudomonas aeruginosa. LWT. 2020;117. [Google Scholar]
- LaSarre B, Federle MJ. Exploiting Quorum Sensing To Confuse Bacterial Pathogens. Microbiol Mol Biol Rev. 2013;77(1):73-111. [Google Scholar] [Crossref]
- Başaran TI, Berber D, Gökalsın B, Tramice A, Tommonaro G, Abbamondi GR. Extremophilic Natrinema versiforme against Pseudomonas aeruginosa quorum sensing and biofilm. Front Microbiol. 2020;11. [Google Scholar]
- Cáceres M, Hidalgo W, Stashenko E, Torres R, Ortiz C. Essential Oils of Aromatic Plants with Antibacterial, Anti-Biofilm and Anti-Quorum Sensing Activities against Pathogenic Bacteria. Antibiotics. 2020;9(4). [Google Scholar] [Crossref]
- Scoffone V, Trespidi G, Chiarelli L, Barbieri G, Buroni S. Quorum Sensing as Antivirulence Target in Cystic Fibrosis Pathogens. Int J Mol Sci. 2019;20. [Google Scholar] [Crossref]
- Jakobsen T, Warming A, Vejborg R, Moscoso J, Stegger M, Lorenzen F. A broad range quorum sensing inhibitor working through sRNA inhibition. Scientific Rep. 2017;7(1):1-2. [Google Scholar] [Crossref]
- Somma A, Moretta A, Canè C, Cirillo A, Duilio A. Antimicrobial and Antibiofilm Peptides. Biomolecules. 2020;10(4). [Google Scholar] [Crossref]
- Koo H, Allan R, Howlin R, Stoodley P, Hall-Stoodley L. Targeting microbial biofilms: current and prospective therapeutic strategies. Nat Rev Microbiol. 2017;15(12):740-55. [Google Scholar] [Crossref]
- Gharieb R, MS, AM, YT. Characterization of two novel lytic bacteriophages for reducing biofilms of zoonotic multidrug-resistant Staphylococcus aureus and controlling their growth in milk. LWT. 2020;124. [Google Scholar] [Crossref]
- Reuter M, Kruger D. Approaches to optimize therapeutic bacteriophage and bacteriophage-derived products to combat bacterial infections. Virus Genes. 2020;56(2):136-49. [Google Scholar] [Crossref]
- Zhang L, Liang E, Cheng Y, Mahmood T, Ge F, Zhou K. Is combined medication with natural medicine a promising therapy for bacterial biofilm infection?. Biomed Pharmacother. 2020;128. [Google Scholar] [Crossref]
- Wang T, Huang W, Duan Q, Wang J, Cheng H, Shao J. Sodium houttuyfonate in vitro inhibits biofilm dispersion and expression of bdlA in Pseudomonas aeruginosa. Mol Biol Rep. 2019;46(1):471-7. [Google Scholar] [Crossref]
- Dey P, Parai D, Banerjee M, Hossain S, Mukherjee S. Naringin sensitizes the antibiofilm effect of ciprofloxacin and tetracycline against Pseudomonas aeruginosa biofilm. Int J Med Microbio. 2020;310(3). [Google Scholar] [Crossref]
- Azman A, Mawang C, Khairat J, AbuBakar S. Actinobacteria—a promising natural source of anti-biofilm agents. Int Microbiol. 2019;22(4):403-9. [Google Scholar] [Crossref]
- Junter G, Thébault P, Lebrun L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016;30:13-25. [Google Scholar] [Crossref]
- Damodaran V, Murthy N. Bio-inspired strategies for designing antifouling biomaterials. Biomater Res. 2016;20(1):1-1. [Google Scholar] [Crossref]
- Pan C, Zhou Z, Yu X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J Orthop Surg Res. 2018;13(1):1-1. [Google Scholar]
- Wang H, Wang M, Strange C, Dong X. Therapeutic effects of adipose-stem cells from diabetic mice for the treatment of Type 2 diabetes. Cytotherapy. 2018;20. [Google Scholar] [Crossref]
- Shin J, Gwak J, Kamarajan P, Fenno J, Rickard A, Kapila Y. Biomedical applications of nisin. J Appl Microbiol. 2016;120(6):1449-65. [Google Scholar] [Crossref]
- Peng X, Zhu L, Wang Z, Zhan X. Enhanced stability of the bactericidal activity of nisin through conjugation with gellan gum. Int J Biol Macromolecules. 2020;148:525-32. [Google Scholar]
- Lu L, Hu W, Tian Z, Yuan D, Yi G, Zhou Y. Developing natural products as potential anti-biofilm agents. Chin Med. 2019;14:1-7. [Google Scholar] [Crossref]
- Abstract
- Introduction
- Anti-biofilm Agents Based on Natural Products
- Quorum Sensing Targeting Strategies
- Phage Therapy
- Combination Therapy
- Anti-biofilm Biomaterial Therapy
- Conclusion and Future Directions
- Conflicts of Interest
- Source of Funding
- References
How to Cite This Article
Vancouver
Raj SS, Thomas NG. Anti biofilm agents – Nature the cure [Internet]. Int Dent J Stud Res. 2021 [cited 2025 Oct 06];9(1):25-32. Available from: https://doi.org/10.18231/j.idjsr.2021.006
APA
Raj, S. S., Thomas, N. G. (2021). Anti biofilm agents – Nature the cure. Int Dent J Stud Res, 9(1), 25-32. https://doi.org/10.18231/j.idjsr.2021.006
MLA
Raj, Sheena S, Thomas, Nebu George. "Anti biofilm agents – Nature the cure." Int Dent J Stud Res, vol. 9, no. 1, 2021, pp. 25-32. https://doi.org/10.18231/j.idjsr.2021.006
Chicago
Raj, S. S., Thomas, N. G.. "Anti biofilm agents – Nature the cure." Int Dent J Stud Res 9, no. 1 (2021): 25-32. https://doi.org/10.18231/j.idjsr.2021.006
