- Visibility 32 Views
- Downloads 9 Downloads
- DOI 10.18231/j.idjsr.2021.035
-
CrossMark
- Citation
Covid tongue: A new indicator of COVID-19 infection-A case report
- Author Details:
-
Mohammed Najmuddin *
-
Halima Almishy
-
Zainab Alhazmi
-
Asayil Jundus
-
Mafaz Gharawi
-
Safeena
Introduction
A handful cases of anonymous viral pneumonia were registered in China in December 2019.[1], [2], [3], [4] The pathogen, a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), was isolated from infected patient’s lower respiratory tract samples, and was referred to as Coronavirus Disease 2019 (COVID-19). [5], [6] It has reached a high rate of infectivity with worldwide estimates of approximately 205,338,159 cases and 4333094 deaths by November 2020. [7], [8]
Transmission of SARS-CoV-2 may take place during the time of incubation period which might be as long as 14 days. [5], [9] Asymptomatic individuals (or peoples within the incubation period) has also been identified as potential carriers; but, the level to which this takes place remains uncertain. [5], [9] It is presumed to transmit by direct person-to-person in proximity of about 2 meter, a range at which an affected individual's respiratory droplets might spread to other people who do not have suitable barriers on coughing, sneezing or speaking. [1], [2], [10], [11], [12], [13], [14], [15] Another route of transmission occurs indirectly when people comes in contact with the salivary droplets which land on various objects, such as the ground and the soil. [10], [13], [16], [17]
There is a varied clinical range of COVID-19 infection, which depends on the host immune reaction. Fever, fatigue, malaise, cough, dyspnea, loss of taste and smell are the most common symptoms. Sever symptoms include advanced pneumonia with respiratory arrest and even death.[1], [5], [7], [11], [18], [19], [20] Elderly patients with or without devastating conditions such as cardiovascular disease, high blood pressure or diabetes are more likely to develop serious infections with higher mortality rates. [1], [3], [7], [11], [21] In Italy, some authors reported cases of dermatological involvement in patients with SARS-CoV-2 infection. Since then many studies have identified dermatological involvement, including lesions ranging from hand and feet affectation in adolescents to vasculitis, rash, urticaria, and varicella-like lesions. [5], [7], [12]
This strain of coronavirus, recently referred to as the hit and run virus, certainly modifies the immune system, triggering distinct changes in response reactions that can act against the host, resulting in autoimmune damage. [12], [13] As far as oral manifestations are concerned the question has to be asked whether this strain has specific abilities to pass through oro-pharyngeal epithelial barriers. [12] Bacterial/fungal coinfection is a proposed etiological hypothesis for oral manifestations linked to COVID-19 that may cause over-prescription of broad-spectrum antibiotics for patients with COVID-19, particularly for those who undergo a longer infection course. [22] The prevalence and effect of bacterial and fungal co-infections has still not been studied, especially in patients with acute respiratory distress syndrome, among various factors contributing to morbidity and mortality in COVID-19 patients. [23]
So far, insufficient importance has been given to the prevalence of fungal infections in patients infected with COVID-19 who may experience lymphocytopaenia, hospitalization in intensive care unit (ICU), broad-spectrum antibiotic and corticosteroid usage, intubation, cytokine storms, and possessing chronic illness which make them seriously immunocompromised. [23], [24], [25], [26], [27], [28]
Here, we present a case associated with oral lesions manifested by this virus. Due to the state of alarm declared on 21 March, we were not able to examine her in our clinic, but we had the possibility of video consultations.
Case Report
A 72-years old female patient was admitted to the hospital complaining of loss of sensation and movement over the right of the body. CT scan showed Cerebrovascular accident (CVA) on the left side of her brain. Very next day of admission she developed dyspnea, with high temperature and fluctuating oxygen level. She was shifted to isolation room suspecting COVID-19 infection. A PCR test was performed on the sample obtained from the nasal swab, which was negative. Patient was discharged but after 6 days she developed severe diarrhea, loss of appetite and vomiting. Chest x-ray revealed bilateral multifocal alveolar opacities. Suspecting COVID-19 another PCR test was performed on the sample obtained from the nasal swab which was positive. Patient had a history of cardiac problem, hypertension and diabetes. She also gave a history of stroke 3 years ago.
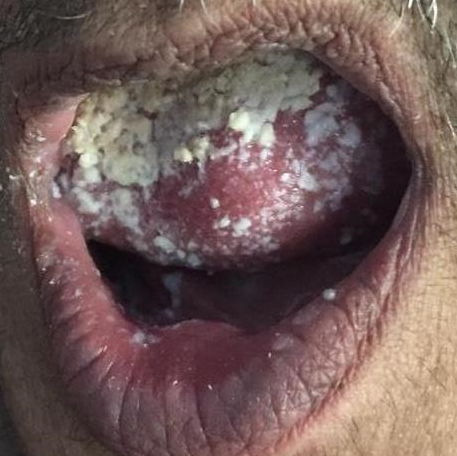
She developed white to whitish yellow creamy plaque like lesions resembling milk curds or cottage cheese over the dorsal surface of tongue.([Figure 1]) It was scrappable leaving behind erythematous bleeding surface. Topical nystatine was applied which lead to the resolution of lesion in 4 days.
Discussion
At the onset of the COVID-19 disease outbreak, the absence of oral participation was thought to be a distinctive characteristic of COVID-19 exanthema compared to other viral exanthemas. SARS-CoV-2 has been identified from patient saliva and the reverse transcriptase-polymerase chain reaction (RT-PCR) from saliva has been shown to be far more sensitive compared to the nasopharyngeal examination.
Essentially, SARS-CoV-2 uses angiotensin converting enzyme 2 (ACE2) receptors to enter cells, primarily those of the lower respiratory system. [11], [13], [29], [30] SARS-CoV-2 may infect nasal and oral mucosal cells along its path to lower respiratory system, which may explain the incidence of odor and taste dysfunction early in the course of the disease. [29], [30], [31], [32] In addition, ACE2 was found in the oral mucosa, notably with an increased concentration mostly over the dorsum of the tongue and salivary glands compared to the buccal or palatal mucosa. [33] Cells with the presence of ACE2 receptors can also become host virus cells and induce inflammatory reactions in associated organs and tissues, such as the mucosa of the tongue and salivary glands.[7] Biadsee and colleagues found that 7% of RT-PCR positive patients had plaque-like alterations on the dorsum of the tongue. [33], [34]
Diverse orofacial manifestations affiliated with COVID-19 have been reported in a variety of studies. Different types of oral mucosal lesions have been identified by Martín Carreras-Presas et al (ulcer, vesicle, bulla, and desquamative gingivitis) that are clinically similar to several other viral mucocutaneous infections, including herpes simplex, herpes zoster, or immunological disorders. [7], [35], [36]
According to a review on oral manifestation published by Iranmanesh et al, the most common sites of involvement in descending order were tongue (38%), labial mucosa (26%), palate (22%), gingiva (8%), buccal mucosa (5%), oropharynx (4%),and tonsil (1%). [33] Because of uncertain treatment options for COVID-19, indirect complex effects, invasive therapeutic methods and multi-drug therapy, SARS-CoV-2 may be anticipated to exacerbate some pathological oral conditions especially in patients with impaired immune systems or who are undergoing long-term pharmacotherapy. [23], [33], [36]
It has been documented that oral candida infection almost always involves a locally or systemically compromised host. Taking into consideration that C. albicans is a member of the oral microbiome and that there were desirable events for its pathological growth, such as the decrease in salivation demonstrated in the patient by the sensation of dry mouth, in addition to the repeated use of antibiotics and a minor predisposing factor such as the female sex and the successful response to nystatin treatment, we can clinically confirm candida infection. [10], [37] In our case the white lesion disappeared after 4 days of topical application of nystatin gel. The possibility of fungal co-infections occurring, especially invasive pulmonary aspergillosis in COVID-19 patients, has been discussed in several studies. In addition, COVID-19 patients are also vulnerable to infections caused by the emerging species, C auris, because of many risk factors. [38], [39] Considering the clinical course, disease progression and severity of COVID-19, most of COVID-19 patients with critically ill conditions inevitably experience at least one of following risk factors, for oropharengial candidiasis (OPC), including lymphocytopaenia, ICU admission, invasive or non-invasive ventilation, corticosteroid and broad-spectrum antibiotic usage or having immunocompromised condition which give them a significantly increased
risk for the development of opportunistic fungal infections. [39], [40], [41], [42]
A few cases of oral manifestations in patients with COVID-19 have been documented in previous literature, but it is still unclear whether it is the direct action of the virus or the result of systemic degradation that increases the risk of opportunistic injury.
Conclusion
Knowledge of clinical signs and symptoms is crucial for early diagnosis, swift treatment and hence better prognosis. Dental professionals can play a significant role not only in preventing the transmission of COVID-19 but also in the early detection and referral of affected patients. We also propose that rigorous reporting of the clinical scenarios of fungal complications such as oropharyngeal candidiasis may help to clarify the current pandemic and the future role of dentists in frontline teams.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Source of Funding
None.
References
- M Najmuddin. Journal of Oral Medicine, Oral Surgery, Oral Pathology and Oral. Radiology 2020. [Google Scholar]
- H Tu, S Tu, S Gao, A Shao, J Sheng. Current epidemiological and clinical features of COVID-19; a global perspective from China. J Infect 2020. [Google Scholar]
- F Zhou, T Yu, R Du, G Fan, Y Liu, Z Liu. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020. [Google Scholar]
- T Singhal. A Review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr 2020. [Google Scholar]
- S Recalcati. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol 2020. [Google Scholar] [Crossref]
- Y H Jin, L Cai, Z S Cheng. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020. [Google Scholar] [Crossref]
- R O C Tapia, A J P Labrador, D M Guimaraes, L H M Valdez. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease?. Spec Care Dent 2020. [Google Scholar] [Crossref]
- Worldmeter. Worldmeter [Homepage on the internet] COVID-19 Coronavirus Pandemic. (Accessed July 29, 2020). 2020. [Google Scholar]
- C Rothe, M Schunk, P Sothmann. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020. [Google Scholar] [Crossref]
- J Corchuelo, F C Ulloa. Oral manifestations in a patient with a history of asymptomatic COVID-19: Case report. Int J Infect Dis 2020. [Google Scholar] [Crossref]
- L Marco. Coronavirus disease 2019 in chronic kidney disease. Clinical Kidney J 2020. [Google Scholar] [Crossref]
- A Dziedzic, R Wojtyczka. The impact of coronavirus infectious disease 19 (COVID-19) on oral health. Oral Dis 2020. [Google Scholar] [Crossref]
- M B Fini. Oral saliva and COVID-19. Oral Oncol 2020. [Google Scholar] [Crossref]
- Yla Kwok, J Gralton, M-L Mclaws. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control 2015. [Google Scholar]
- J Rubens, P Karakousis, J Sanjay. Stability and viability of SARS-CoV-2. N Engl J Med 2020. [Google Scholar] [Crossref]
- M B Fini. What dentists need to know about COVID-19. Oral Oncol 2020. [Google Scholar] [Crossref]
- Z Khurshid, F Y Asiri, H Al Wadaani, . Human saliva: non-invasive fluid for detecting novel Coronavirus (SARS-CoV-2). Int J Environ Res Public Health 2020. [Google Scholar] [Crossref]
- H Harapan, N Itoh, A Yufika, W Winardi, S Keam, H Te. Coronavirus disease 2019 (COVID-19): A literature review. J Infect Public Health 2020. [Google Scholar]
- L Wang, Y Wang, D Ye, Q Liu. Review of the 2019 novel coronavirus (SARS- CoV-2) based on current evidence. Int J Antimicrob Agents 2020. [Google Scholar] [Crossref]
- H Zhang, H B Li, J R Lyu, X M Lei, W Li, G Wu. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis 2020. [Google Scholar] [Crossref]
- L Fu, B Wang, T Yuan. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect 2020. [Google Scholar] [Crossref]
- A Riad, A Gad, B Hockova, M Klugar. Abanoub Riad et al, Oral candidiasis in non-severe COVID-19 patients: call for antibiotic stewardship. Oral Surg 2021. [Google Scholar] [Crossref]
- M Salehi. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses . 2020. [Google Scholar] [Crossref]
- J-P Gangneux, M-E Bougnoux, E Dannaoui, M Cornet, Z J Ralph. Invasive fungal diseases during COVID-19: We should be prepared. J Mycol Med 2020. [Google Scholar] [Crossref]
- W-J Guan, Z-Y Ni, Y U Hu. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. [Google Scholar] [Crossref]
- C Huang, Y Wang, X Li. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet 2020. [Google Scholar]
- . World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (2019-nCoV) infection is suspected: Interim Guidance. 2020. WHO Reference number: WHO/2019- nCoV/clinical. 2020. [Google Scholar]
- A Akpan, R Morgan. Oral candidiasis. Postgrad Med J 2002. [Google Scholar]
- H Zhang, J M Penninger, Y Li, N Zhong, A S Slutsky. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 2020. [Google Scholar] [Crossref]
- A Giacomelli, L Pezzati, F Conti, D Bernacchia, Mo Siano, L Oreni. Self-reported Olfactory and Taste Disorders in Patients With Severe Acute Respiratory Coronavirus 2 Infection: A Cross-sectional Study. Clin Infect Dis 2020. [Google Scholar] [Crossref]
- F A C Garcia de Sousa, T C Paradella. Considerations on oral manifestations of COVID-19. J Med Virol 2020. [Google Scholar] [Crossref]
- X H Yang, W Deng, Z Tong, Y X Liu, L F Zhang, H Zhu. Mice transgenic from human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comput Med 2007. [Google Scholar]
- B Iranmanesh, M Khalili, R Amiri, H Zartab, M Aflatoonian. Oral manifestations of COVID-19 disease: A review article. Dermatol Ther 2020. [Google Scholar] [Crossref]
- A Biadsee, A Biadsee, F Kassem, O Dagan, S Masarwa, Z Ormianer. Olfactory and oral manifestations of COVID-19: sex-related symptoms-a potential pathway to early diagnosis. Otolaryngol Head Neck Surg 2020. [Google Scholar]
- C M Carreras-Presas, J Amaro Sánchez, A F López-Sánchez, E Jané-Salas, M L S Pérez. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis 2020. [Google Scholar] [Crossref]
- J A Dos Santos, A G C Normando, R L Carvalho da Silva, A C Acevedo, G De Luca Canto, N Sugaya. Oral Manifestations in Patients with COVID-19: A Living Systematic Review. J Dent Res 2021. [Google Scholar] [Crossref]
- D J Zegarelli. Fungal infections of the oral cavity. Otolaryngol Clin North Am 1993. [Google Scholar]
- A Chowdhary, A Sharma. The lurking scourge of multidrug resistant Candida auris in times of COVID-19 pandemic. J Glob Antimicrob Resist 2020. [Google Scholar] [Crossref]
- M Salehi, K Ahmadikia, S Mahmoudi. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: Species identification and antifungal susceptibility pattern. Mycoses 2020. [Google Scholar] [Crossref]
- A Riad, M Klugar, M Krsek. COVID-19 related oral manifestations, early disease features?. Oral Dis 2020. [Google Scholar] [Crossref]
- M Salehi, K Ahmadikia, S Mahmoudi, S Kalantari, S Jamalimoghadamsiahkali, A Izadi. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses 2020. [Google Scholar] [Crossref]
- R Abanoub, A Gad, B Hockova, M Klugar. Letter to the editor Oral candidiasis in non-severe COVID-19 patients: call for antibiotic stewardship. Oral Surg 2020. [Google Scholar] [Crossref]
